Method for synthesizing m-phenoxybenzoic cyanohydrin ester succinate
A technology of m-phenoxybenzocyanohydrin succinate and m-phenoxybenzocyanhydrin, which is applied in chemical instruments and methods, preparation of carboxylic acid nitriles, preparation of organic compounds, etc., to achieve simple synthesis steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
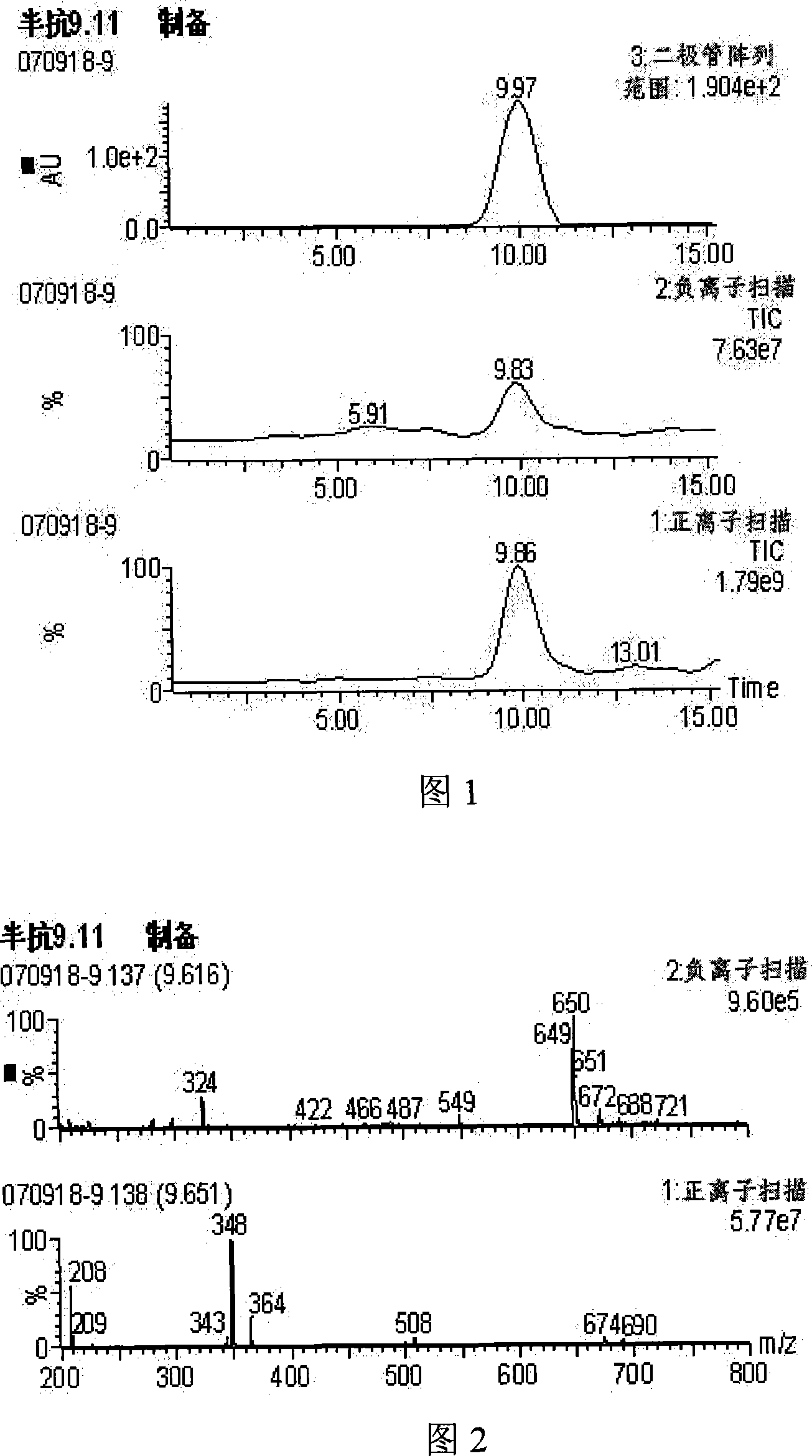
Image
Examples
Embodiment 1
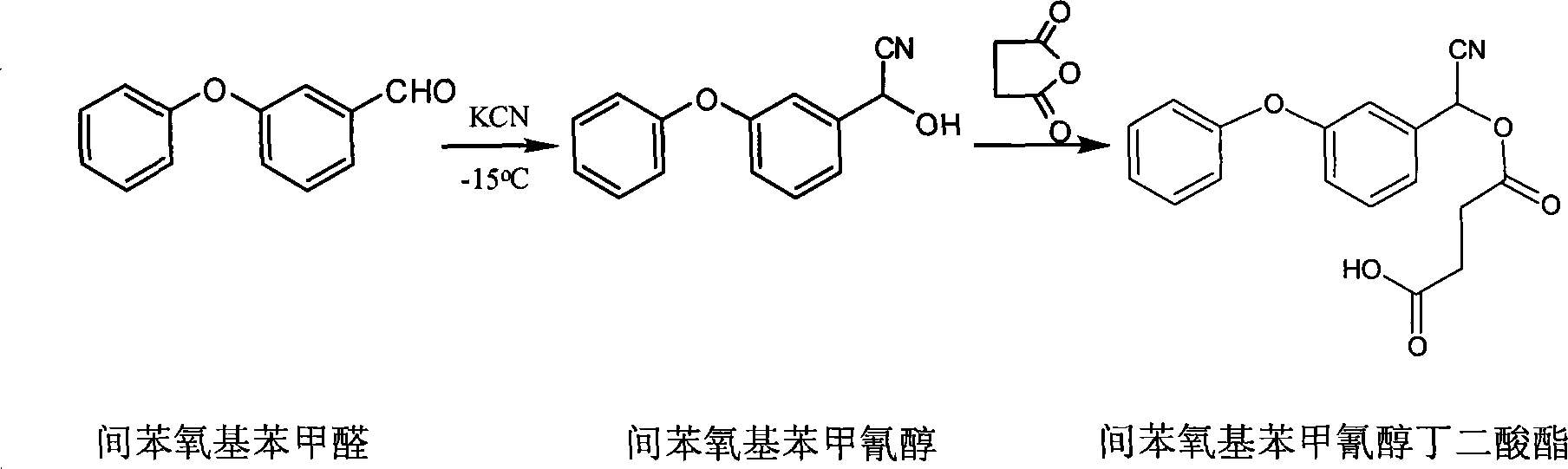
[0019] (1) the preparation of m-phenoxybenzoyl cyanohydrin:
[0020] Add 0.3g (4.6mmol) potassium cyanide and 1ml distilled water in a small reaction flask equipped with a stirrer, cool to -15°C under stirring, add m-phenoxybenzaldehyde 0.8g (4.0mmol), in 1 ml (5.3 mmol) of 40% sulfuric acid was added dropwise within 10 minutes. After continuing to stir for 15 minutes, it was extracted with 40 ml of carbon tetrachloride. After the extract was dried with anhydrous sodium sulfate, the solvent was removed to obtain 0.64g (2.9mmol) of m-phenoxybenzyl cyanohydrin, n D = 1.5823.
[0021] (2) Synthesis of the derivative m-phenoxybenzocyanhydrin succinate of m-phenoxybenzocyanhydrin:
[0022] Dissolve the obtained m-phenoxybenzocyanohydrin in 10 mL of anhydrous pyridine, then add 0.35 g (3.5 mmol) of succinic anhydride, heat in a sand bath to 130 ° C, reflux for 3 hours under magnetic stirring, and then cool with ice water The reactor was removed from the solvent, and then washed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com