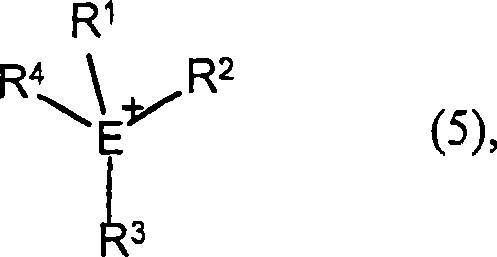
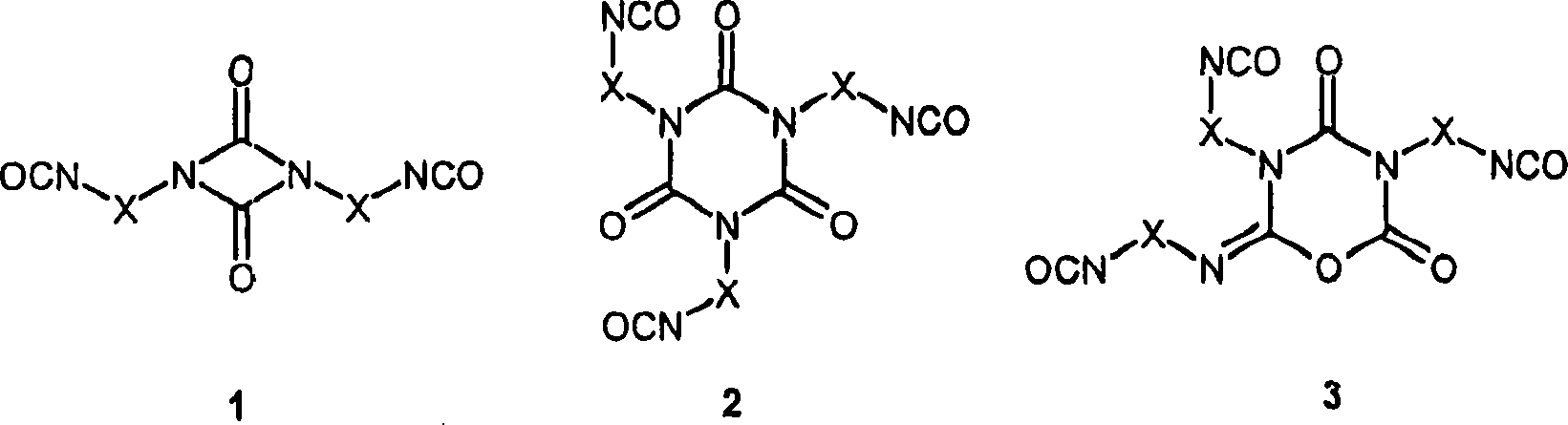
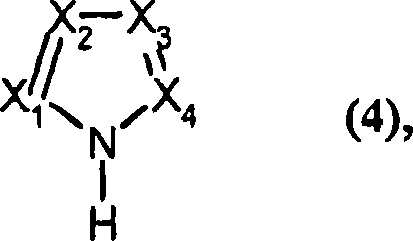Method for preparing polyisocyanic acid ester
A technology of diisocyanate and polyisocyanate, applied in organic chemical methods, polyurea/polyurethane coatings, coatings, etc., can solve problems such as slow reaction process and disadvantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0063] All percentages, unless otherwise indicated, are to be understood as percentages by weight.
[0064] The NCO content of the resins described in the inventive and comparative examples was determined by titration according to DIN53185.
[0065] The dynamic viscosity of the polyisocyanate was measured at 23°C using a Haake VT550 viscometer with a PK100 plate / cone measurement setting. Measurements at different shear rates ensured that both the rheology of the described polyisocyanate mixtures according to the invention and that of the comparative products corresponded to the behavior of an ideal fluid. Therefore, it is no longer necessary to give the shear rate.
[0066] The mol % or the molar ratio of the different types of structures to each other is given according to the results of NMR spectroscopy. Unless stated otherwise, reference is always made to the sum of the various types of structures resulting from the modification (oligomerization) of the hitherto free NCO ...
example 1~3
[0082] Example 1~3: the oligomerization reaction of the present invention
[0083] General procedure
[0084] The rolled flange container with septum seal was evacuated twice and filled with argon. In each case, 5 mL of diisocyanate were introduced by means of a syringe into the container thus prepared, and the corresponding amount of catalyst solution was then added with stirring. See Tables 1 and 2 for catalyst numbering, the "mol %" figures in Tables 3-6 refer in each case to the target amount of diisocyanate and catalyst used to achieve the conversions obtained in the respective experiments. The resulting reaction mixture is reacted at the desired temperature using an oil bath or a stirring heating block (for example, Variomag reaction block, type 48.2 / RM, manufactured by H&P). After the end of the reaction—either by the specified reaction time or by the arrival of a specified viscosity—an aliquot (20-40 mg) of the reaction mixture was dissolved in 3 mL of chloroform a...
example 4
[0109] The inventive preparation of HDI polyisocyanate
[0110] 1680g (10mol) of freshly distilled HDI was first stirred in a 3-neck flask stirring device at 60°C under reduced pressure (0.1mbar) for 1h in order to drive out the dissolved gas, then covered with dry nitrogen and heated at 60°C under stirring Catalyst solution 13 was added dropwise and mixed until the reaction started as indicated by a temperature rise of 1-2°C. By sporadically adding further catalyst (total: 4.6 g catalyst solution = 0.046 mol% catalyst based on the amounts of HDI and 1,2,3-triazolyltetradecyl(trihexyl)phosphonium salt), the reaction was Within 1h, under the temperature condition of the mixture of 60-70℃ to the required conversion rate, the required conversion rate is determined by the refractive index n D 20 detection. in n D 20 =1.4668, the further reaction was terminated by adding 1.9 g of a 42% strength p-toluenesulfonic acid solution in isopropanol. The crude product thus obtained wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com