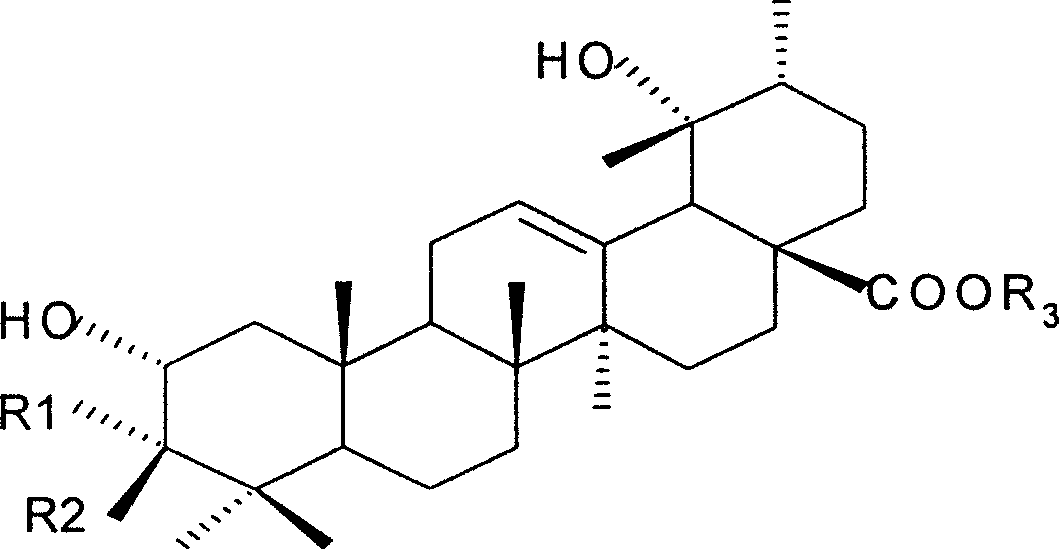
Medicine use of potentilla plants total-triterpene extract
A technology of Potentilla genus and total triterpenes, applied in the field of medicine, can solve problems such as limited therapeutic effect, expensive insulin therapy, and reduced insulin therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
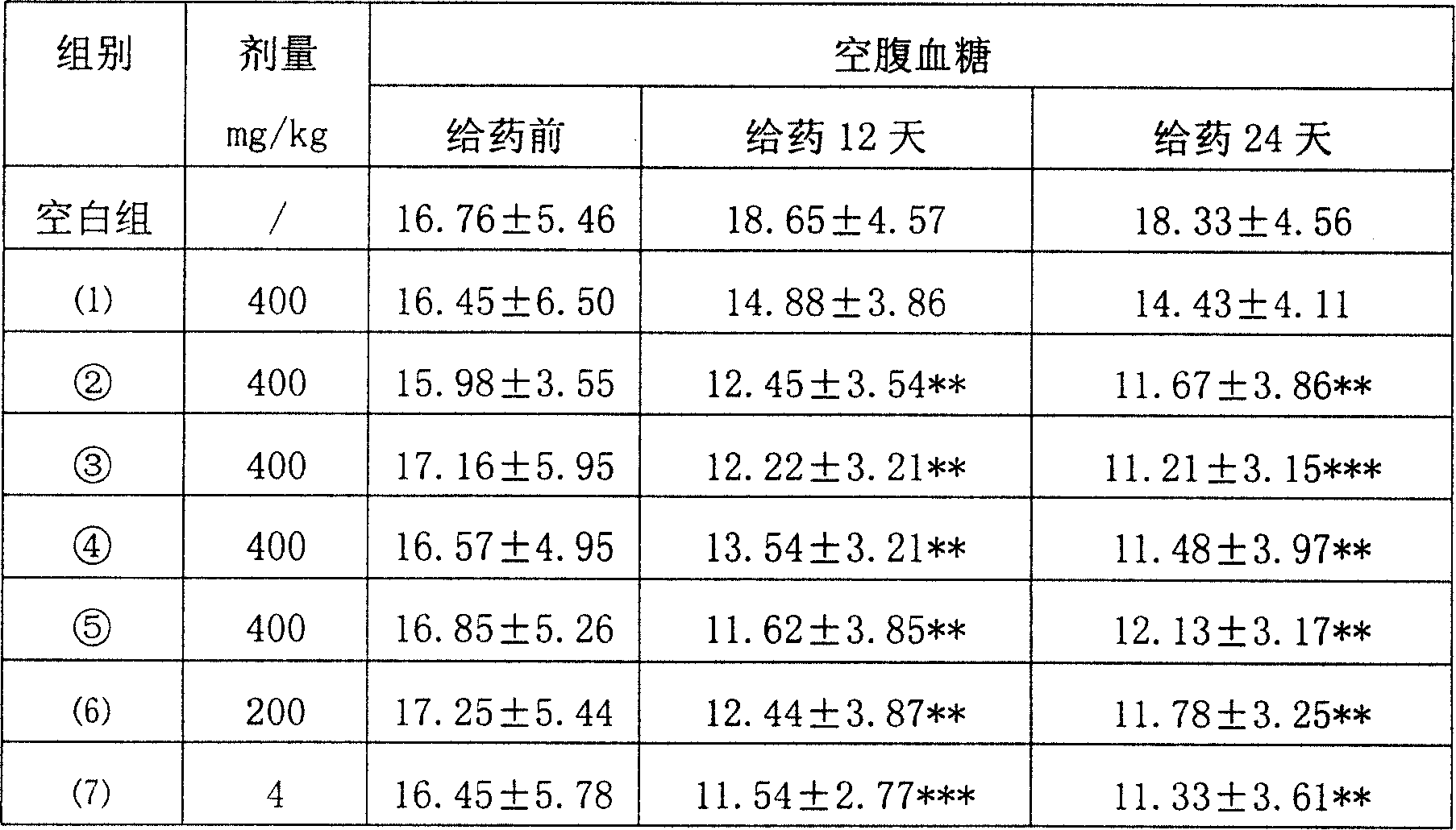
[0021] [Example 1] Effect test of medicine of the present invention on fasting blood glucose in male KKAy diabetic mice
[0022] Test samples: (1) Potentilla water extract; ②Potentilla triterpenoid extract, with a total triterpene content of 55.3%; The total triterpene extract of Fenbaicao, with a total triterpene content of 53.7%; ⑤ the total triterpene extract of Jinlaomei, with a total triterpene content of 66.1%; (6) rosiglitazone; (7) rosiglitazone (positive control drug, ROS).
[0023] Male KKAy diabetic mice (spontaneous diabetic mice, a good animal model of type II diabetes) were adapted to measure random and fasting blood glucose after 2 weeks. According to the measurement results, the mice were divided into 8 groups, 10 in each group, and the normal feeding group ( Blank control group), 4 groups (②, ③, ④, ⑤) of total triterpene extracts of Potentilla plants, and 3 groups of drug control groups ((1), (6), (7)). Dosing according to Table 1, once a day, for 24 consecu...
Embodiment 2
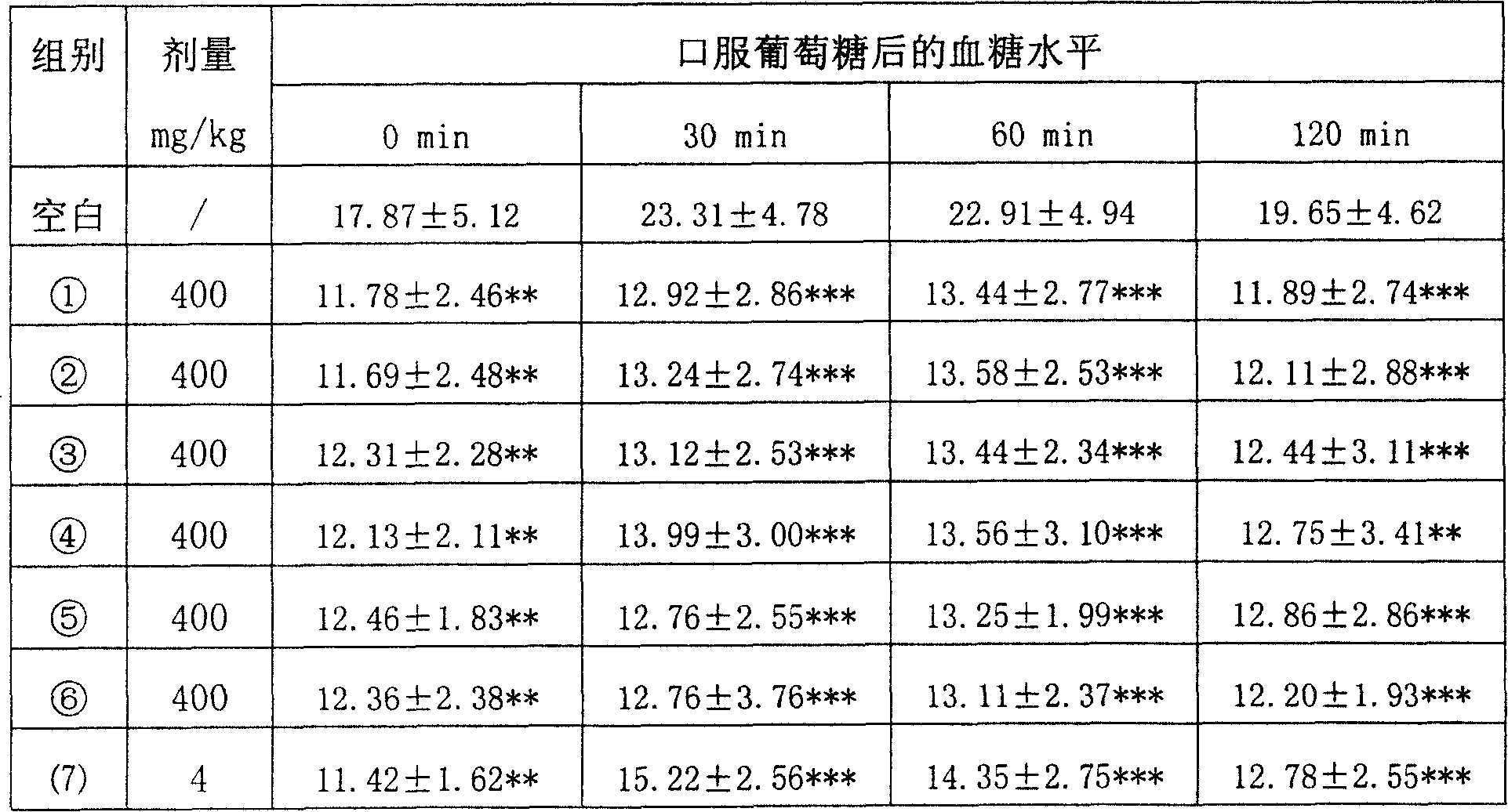
[0030] [Embodiment two] the influence test of medicine of the present invention to the glucose tolerance of male KKAy diabetic mice
[0031] Test samples: ①The total triterpene extract of Potentilla yunnanensis, with a total triterpene content of 51.7%; The total triterpene content is 50.3%: ④The total triterpene extract of Potentia berry leaves, the total triterpene content is 40.1%; The total triterpene extract of Lingcai, the total triterpene content is 35.0%; (7) Rosiglitazone (positive control drug, ROS).
[0032] Male KKAy diabetic mice were adapted to measure random and fasting blood glucose after 2 weeks of adaptation. According to the measurement results, the mice were divided into 8 groups with 10 mice in each group. Group 6 groups (①, ②, ③, ④, ⑤, ⑥), drug control group 1 group ((7)). Administration according to Table 2, once a day, continuous administration for 24 days. After the administration, a glucose tolerance test was carried out, and 2.5 g / kg of glucose wa...
Embodiment 3
[0039] [Embodiment three] Effect test of medicine of the present invention on insulin tolerance in male KKAy diabetic mice
[0040] Test samples: ①The total triterpene extract of Prunus chinensis; the total triterpene content is 35.5%; The total triterpene content is 52.3%; ④The total triterpene extract of P. chinensis, the total triterpene content is 53.7%; Extract, total triterpene content 56.2%; (7) Rosiglitazone (positive control drug, ROS).
[0041] Male KKAy diabetic mice were adapted to measure random and fasting blood glucose after 2 weeks of adaptation. According to the measurement results, the mice were divided into 8 groups with 10 mice in each group. Group 6 groups (①, ②, ③, ④, ⑤, ⑥), drug control group 1 group ((7)). Administration according to Table 2, once a day, continuous administration for 24 days. At the end of administration, the blood glucose values before and after subcutaneous injection of insulin were measured respectively.
[0042] Table 3 Effect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com