Esterification catalysts and esterification process of organic acid
An esterification catalyst and catalyst technology, applied in physical/chemical process catalysts, organic chemistry, chemical instruments and methods, etc., can solve the problems of large amount of ionic liquid, large amount of organic solvents, high energy consumption, etc., to simplify the esterification reaction Process, high yield of esterified product, effect of prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
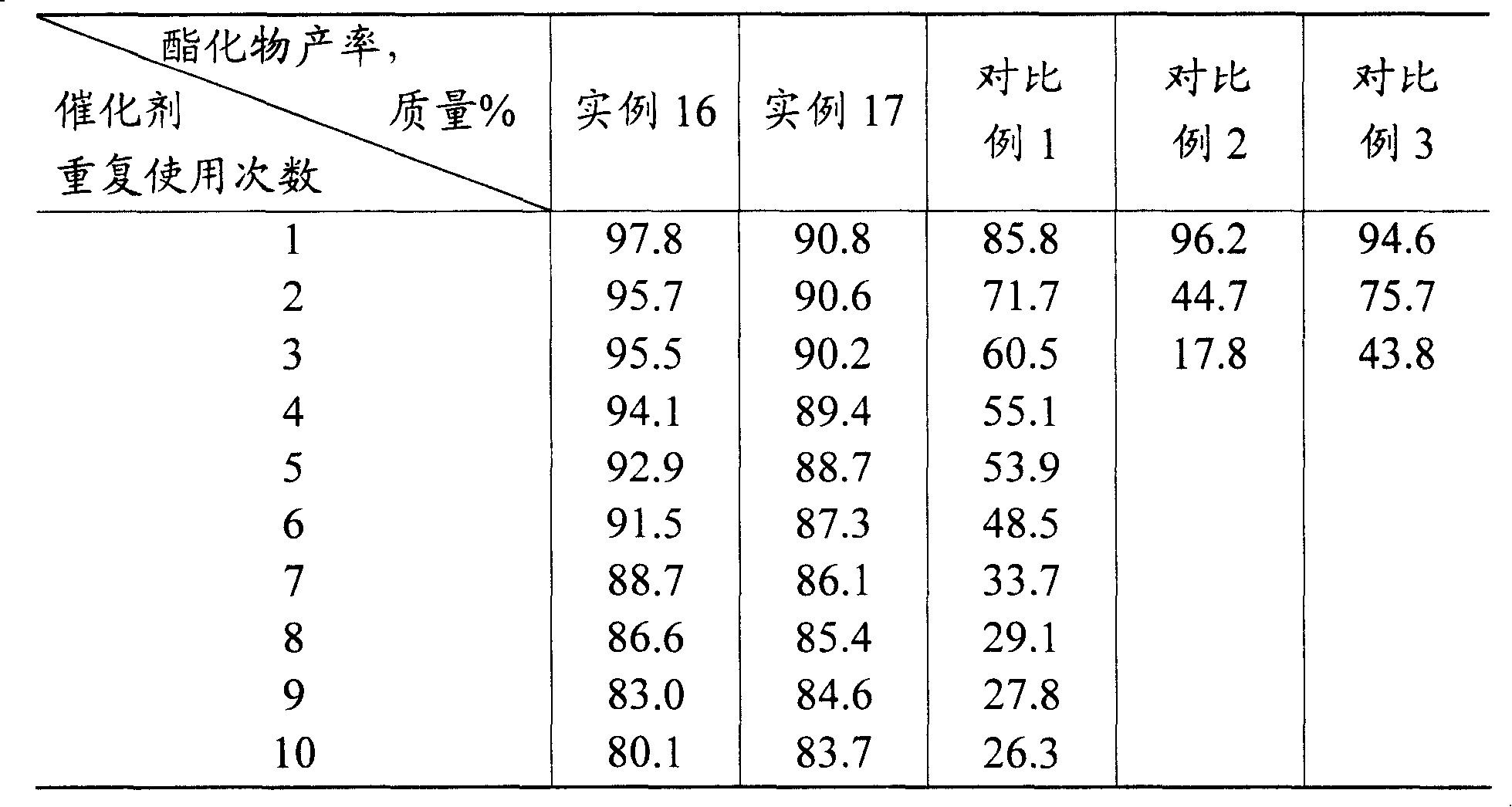
Examples
example 1
[0023] (1) Preparation of ionic liquid
[0024]Put 1mol of 1-methylimidazole into a three-necked round-bottomed flask, add tetrafluoroboric acid (concentration: 40wt%) while stirring in an equimolar ratio, and control the reaction temperature not higher than 40°C, continue stirring for 1h to obtain yellow liquid. Remove excess water by distillation under reduced pressure at 70°C to obtain ionic liquid 1-methylimidazolium tetrafluoroborate [Hmim] + BF 4 - .
[0025] (2) carry out esterification reaction
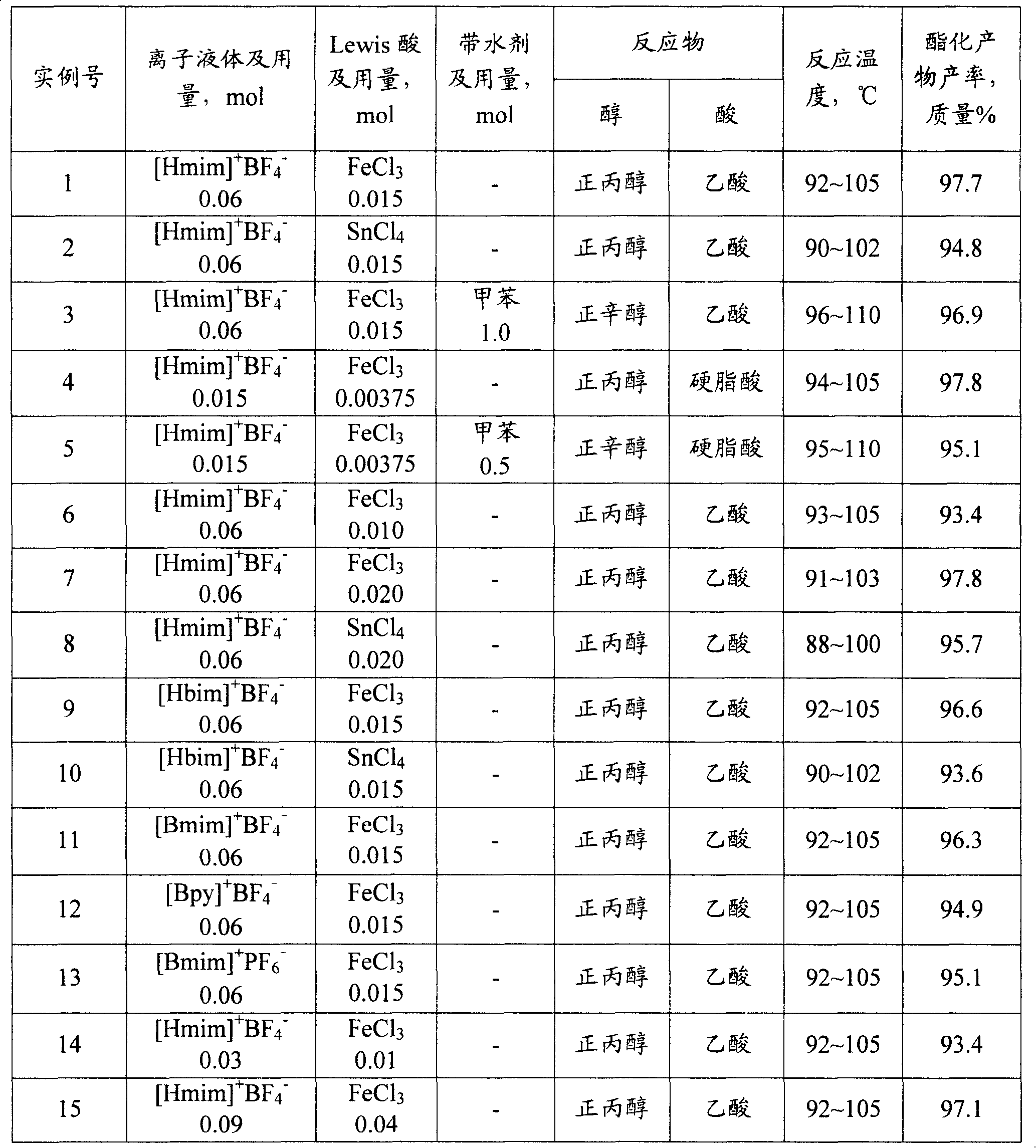
[0026] 0.015molFeCl 3 With 0.06mol ionic liquid [Hmim] + BF 4 - After mixing evenly, put it into a 500mL three-necked bottle together with 1mol acetic acid and 1mol n-propanol, install a water separator and a temperature sensor, install a reflux condenser on the water separator, and heat until the reactant boils while stirring to start the reaction (reaction temperature 92~105℃). The azeotrope formed during the reaction is distilled from the three-necked flask, conde...
example 2
[0028] Carry out esterification reaction by the method for example 1, difference is (2) step with 0.015molSnCl 4 instead of FeCl 3 With 0.06mol ionic liquid [Hmim] + BF 4 - Mix to make a catalyst, and the results of the esterification reaction are shown in Table 1.
example 3
[0030] Carry out esterification reaction by the method for example 1, difference is (2) in the step with 0.015molFeCl 3 With 0.06mol ionic liquid [Hmim] + BF 4 - After mixing evenly, put 60.0g (1mol) of acetic acid, 130.0g (1mol) of n-octanol and 92.0g (1mol) of toluene into the reaction bottle for esterification reaction. After almost no water is separated from the reflux, use vacuum distillation The method separates toluene, esterification product n-octyl acetate and unreacted raw materials from the catalyst, and the results of the esterification reaction are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com