Method for rapidly detecting grouper irido virus
An iridescent virus, detection method technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problem of complex LAMP primer design and other problems, and achieve the effect of rapid detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Establishment of LAMP detection method
[0062] 1. SGIVORF-014L cloning and plasmid template construction
[0063] 1.1 Materials and methods
[0064] 1.1.1 Materials
[0065] 1.1.1.1 Viruses and Cells
[0066] Grouper iridovirus SGIV (Singapore grouper iridovirus SGIV) is preserved by our laboratory.
[0067] GP cells (Grouper embryo cells) were subcultured and preserved by our laboratory.
[0068] 1.1.1.2 Plasmid vectors and recipient strains
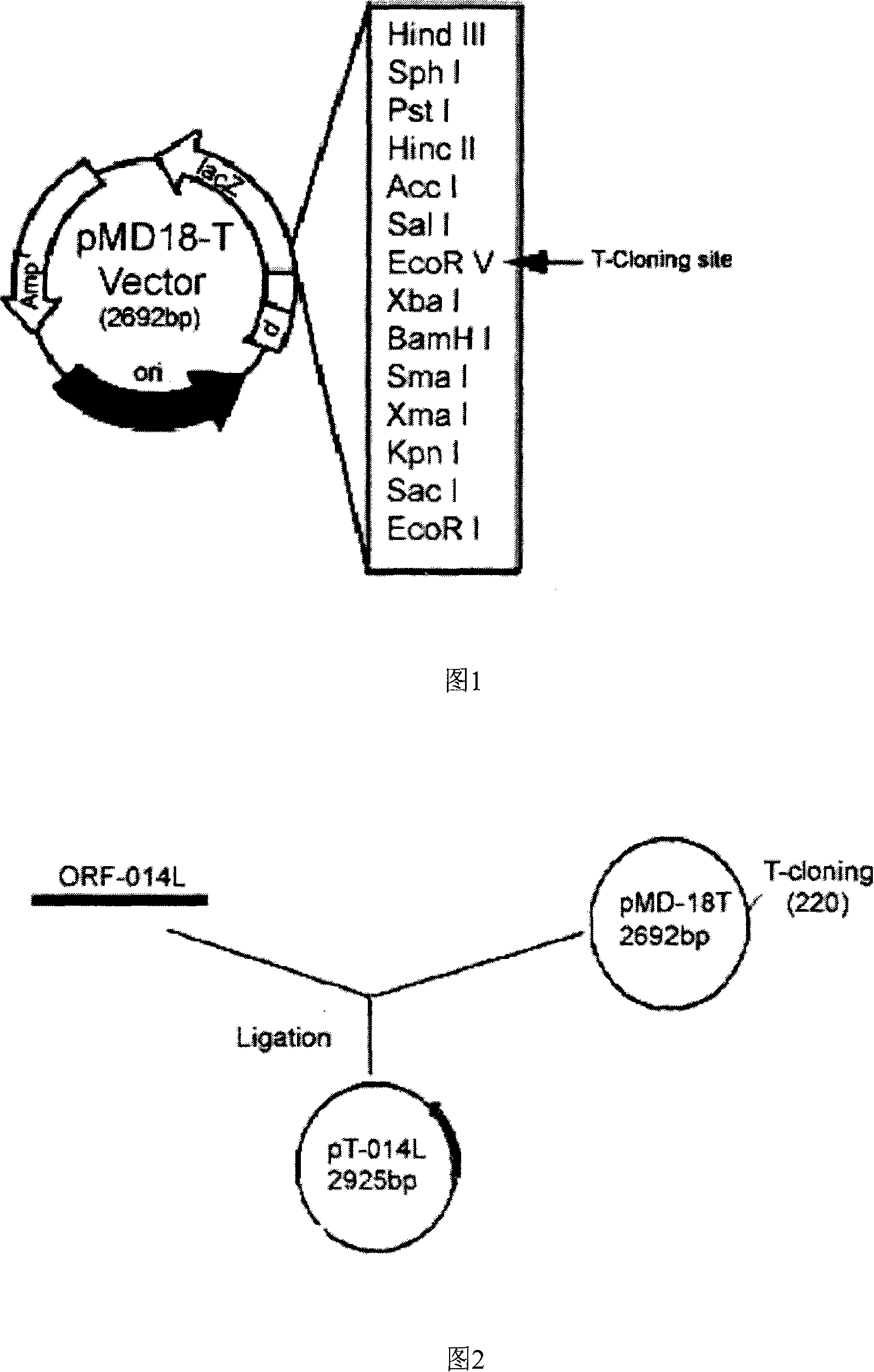
[0069] Plasmid vector: pMD18-T (Figure 1), purchased from TaKaRa Company.
[0070] Recipient bacteria: Escherichia coli strain JM109, genotype: recA supE44 endA1 hsdR17 gyrA96recA1 thiΔ(lac-proAB)F'[traD36proAB+lacI1 lacZΔM15.
[0071] 1.1.1.3 Medium
[0072] LB liquid medium: 10g of trytone; 5g of yeast extract; 10g of NaCl, add 800mL of distilled water, adjust the pH to 7.2-7.4 with 5M NaOH, dilute to 1000mL, aliquot, autoclave, 4 Store at ℃.
[0073] LB solid medium: Add imported agar powder to LB liquid me...
Embodiment 2
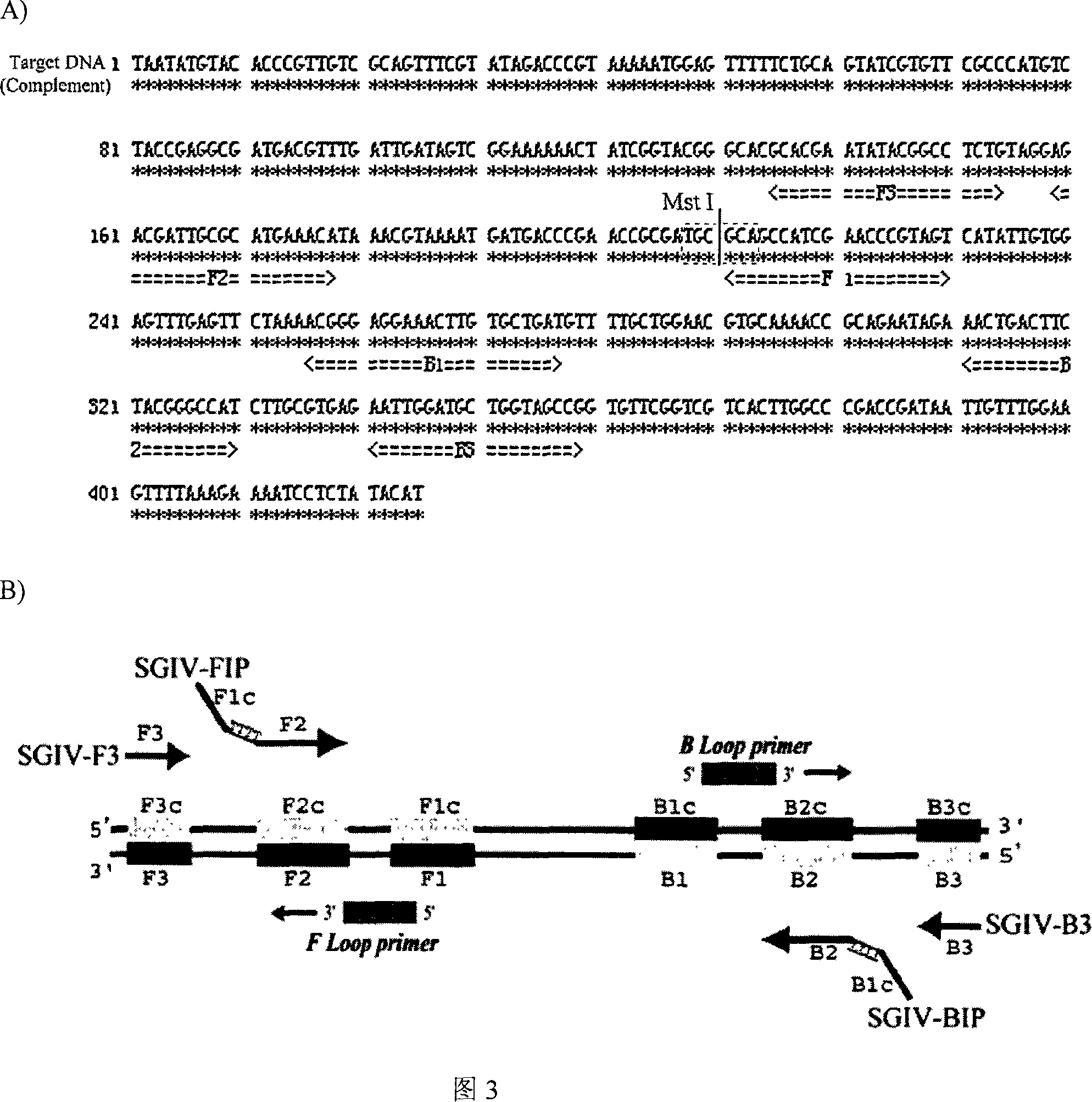
[0290] LAMP primers were designed according to the sequence of SGIV ORF-014L, and the primers were designed and screened using Primer Explorer V3 software (Fujitsu). The primer sequences obtained were:
[0291] F3: GCACGAGTATACGGCCTCTG
[0292] B3: CGGCTACCAGCATCCAAT
[0293] FIP:
[0294] GACTACGGGTTCGATGGCTGC-TTTT-AGACGATTGCGCATGAAACA
[0295] BIPs:
[0296] ACGGGAGGAAACTTGTGCTGAT-TTTT-ATGGCCCGTAGAAGTCAGT
[0297] LF: TCGCGGTTCGGGTCATCAT
[0298] LB: TGCTGGAACGTGCAAAACCG
[0299] According to the selected primers, the 145-359bp fragment of SGIV ORF-014L sequence was successfully amplified, and the region was 215bp in total.
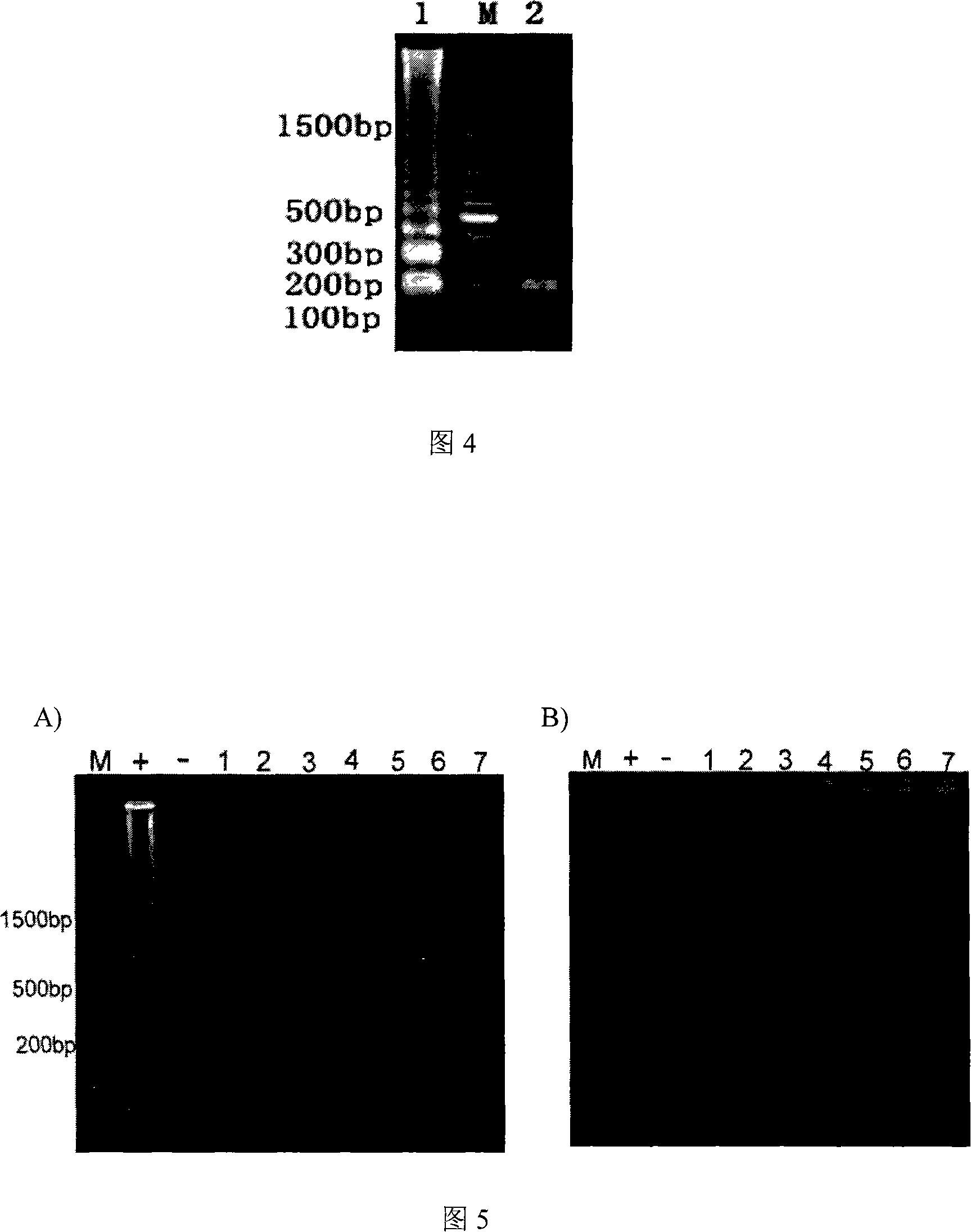
[0300] The LAMP product has a Ladder-like structure in the gel. In order to determine the specificity of the amplified product, the restriction endonuclease MstI was used to digest and identify the protein between F2 and F1c. site, the resulting fragments were consistent with the predicted sizes (218bp and 187bp) (Fig. 4). The specificity of t...
Embodiment 3
[0308] Example 3 LAMP amplification technology detects GP cells infected by SGIV
[0309] SGIV infected GP cells, and after more than 70% of GP cells appeared CPE, the infected cells and healthy control cells were collected. Whole-genome DNA was extracted for LAMP amplification and conventional PCR amplification. Plasmid pT-014L was used as a positive control, and autoclaved water was a negative control.
[0310] The detection of SGIV by LAMP amplification has good specificity, which is consistent with the results of conventional PCR detection, as shown in Figure 8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com