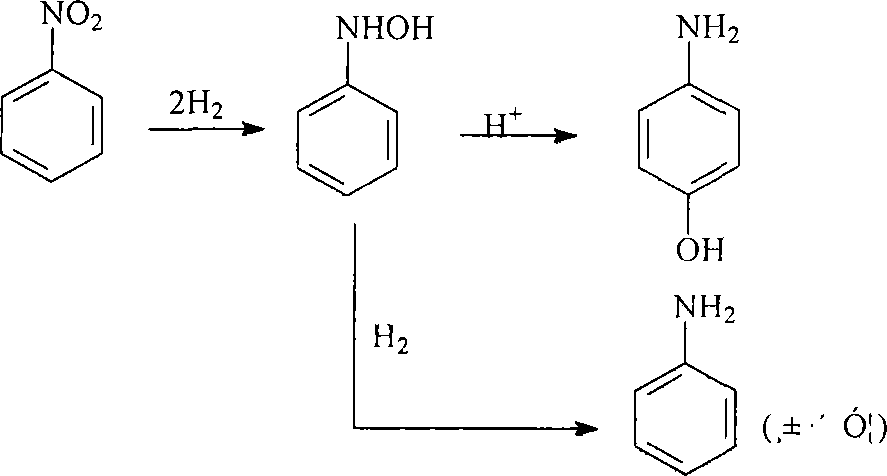
Catalyzer for synthesizing p-aminophenol by nitrobenzene catalytic hydrogenation as well as method for preparing and applying the same
A technology for catalytic hydrogenation of p-aminophenol, which is applied in the preparation of organic compounds, preparation of aminohydroxyl compounds, organic compound/hydride/coordination complex catalysts, etc., can solve environmental pollution of wastewater discharge, harsh reaction conditions, equipment Corrosion and other problems, to achieve the effect of good stability, high mechanical strength and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of room temperature ionic liquid N,N,N-trimethyl-N-sulfobutyl-ammonium bisulfate:
[0044] In the first step, take an aqueous solution of trimethylamine containing 0.50 mol of trimethylamine and 0.55 mol of 1,4-butane sultone, put them in a 250ml three-necked flask, and react at room temperature to 60°C for 8 to 48 hours. After the reaction solution was taken out, water was distilled off under reduced pressure to obtain a white zwitterionic solid, which was washed sequentially with absolute ethanol, toluene and anhydrous ether, and then vacuum-dried at 80°C to constant weight to obtain amphoteric Ion N,N,N-trimethyl-N-sulfobutylammonium, the weight content of trimethylamine in the trimethylamine aqueous solution is more than or equal to 33%.
[0045] In the second step, take 0.50mol of the zwitterion N,N,N-trimethyl-N-sulfobutylammonium prepared in the first step and put it into a four-necked flask, and slowly drop the Add 0.48-0.52 mol of concentrated sulfur...
Embodiment 2
[0048] In the first step, weigh 10ml (9.356g) of tetraethyl orthosilicate and place it in a reactor, add 2ml of absolute ethanol and 30ml of deionized water, and stir evenly in a 90°C water bath;
[0049] In the second step, take by weighing the room temperature ionic liquid N, N, N-trimethyl-N-sulfobutyl-ammonium bisulfate 0.142g and chloroplatinic acid 0.754mg (equivalent to metal platinum is 0.284mg ), which is added to the reactor of the first step, stirred until a gel is formed, and then aged at room temperature for 24 hours;
[0050] In the third step, the material obtained in the second step is vacuum-dried at 100° C. for 0.5 hours;
[0051] In the fourth step, the dried substance in the third step is roasted in a muffle furnace at 120° C. for 5 hours in a nitrogen atmosphere or an air atmosphere;
[0052] In the fifth step, the material obtained in the fourth step is placed in a tubular reactor, and the volume ratio is N 2 :H 2 = 2:1 N 2 and H 2 The mixed gas was ...
Embodiment 3
[0055] In the first step, weigh 10ml (9.356g) of ethyl orthosilicate and place it in a reactor, add 40ml of absolute ethanol and 4ml of deionized water, and stir evenly in a water bath at 30°C;
[0056] In the second step, take by weighing the room temperature ionic liquid N, N, N-trimethyl-N-sulfobutyl-ammonium bisulfate 2.7g and chloroplatinic acid 0.215g (equivalent to metal platinum is 0.081g ), adding it into the reactor of the first step, stirring until a gel is formed, and aging at 100° C. for 10 hours;
[0057] In the third step, the material obtained in the second step is vacuum-dried at 30° C. for 24 hours;
[0058] In the fourth step, the dried substance in the third step is roasted in a muffle furnace at 250° C. for 1 hour in a nitrogen atmosphere or an air atmosphere;
[0059] In the fifth step, the material obtained in the fourth step is placed in a tubular reactor, and the volume ratio is N 2 :H 2 = 2:1 N 2 and H 2 The mixed gas is used as reducing gas, red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com