Amido complex compound and preparation method and usage
A complex and amino technology, applied in the fields of storage and transportation, separation of ammonia gas, and purification, can solve the problems of easy leakage of ammonia gas, harm to health, pressure rise, etc., to improve conversion efficiency, fast separation speed, and increase ammonia net worth effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Formulated 15% by weight of LiF, LiCl, LiBr, MgF 2 , MgCl 2 , CaCl 2 , Ca(NO 3 ) 2 , CaSO 4 , AlCl 3 , Al(NO 3 ) 3 , FeCl 3 , CoCl 2 , Co(NO 3 ) 2 , MnCl 2 , NiCl 2 , CuCl 2 , CuSO 4 , ZnCl 2 , ZnSO 4 , BaCl 2 , BaSO 4 , SnCl 2 , MgCl 2 -CaCl 2 , MgF 2 -CaCl 2 , CaCl 2 -Ca(NO 3 ) 2 , CaCl 2 -AlCl 3 , NiCl 2 -CuCl 2 , Co(NO 3 ) 2 -Zn(NO 3 ) 2 , MgCl 2 -CaCl 2 -AlCl 3 , MgCl 2 -CaCl 2 -CuCl 2 , CaCl 2 -BaCl 3 -ZnCl 2 , Cu(NO 3 ) 2 -Co(NO 3 ) 2 -Zn(NO 3 ) 2 , MgCl 2 -CaCl 2 -CaSO 4 -ZnSO 4 , MgCl 2 -CaCl 2 -CuCl 2 -ZnCl 2 Methanol solution, under the condition of reaction temperature of 30°C and constant stirring, pass ammonia gas into the solution to form a saturated amino complex solution; filter the saturated amino complex solution to obtain amino complex precipitate , and wash the precipitate with methanol solution saturated with ammonia; the washed amino complex precipitates under the protection of an inert gas,...
Embodiment 2
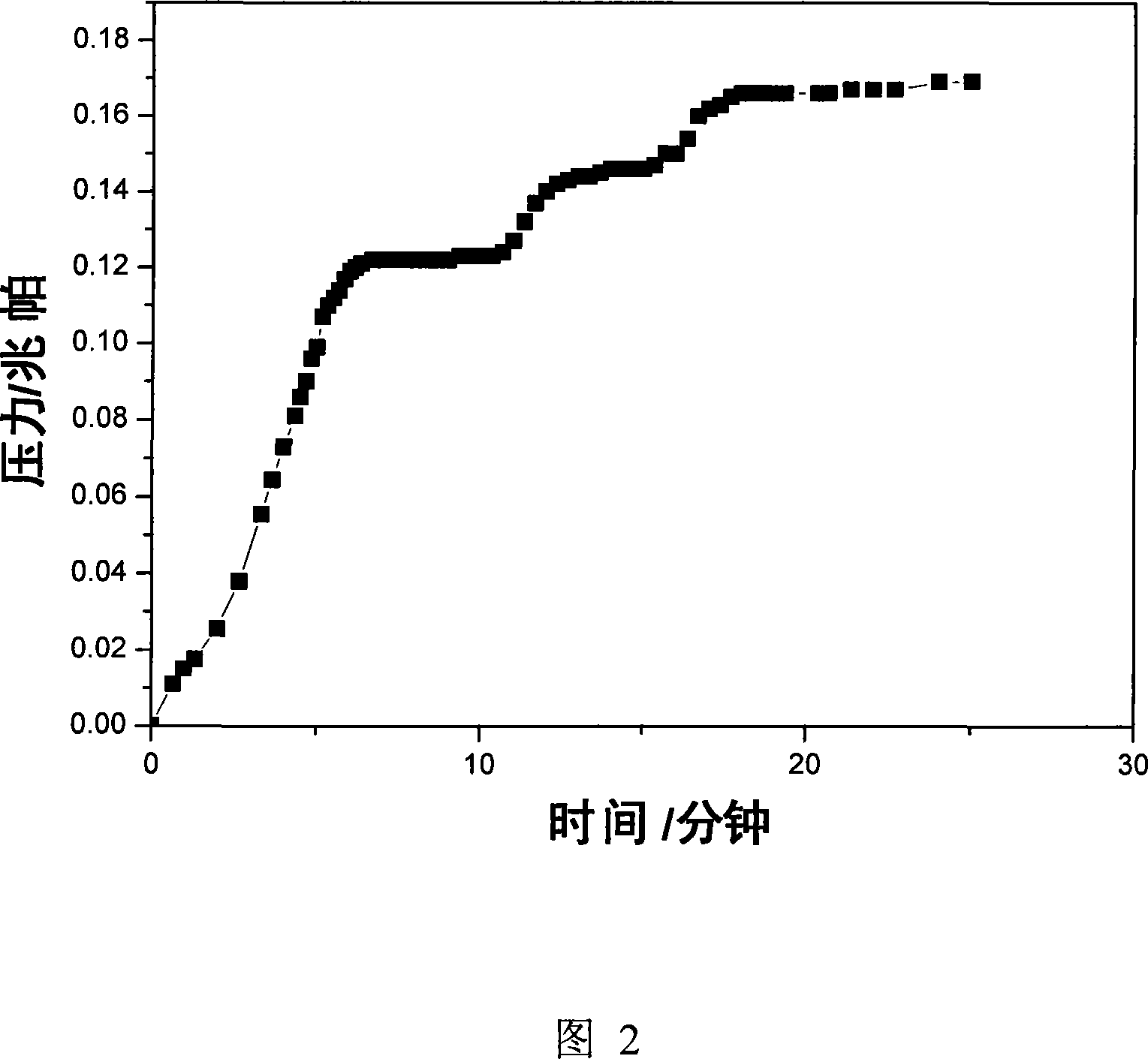
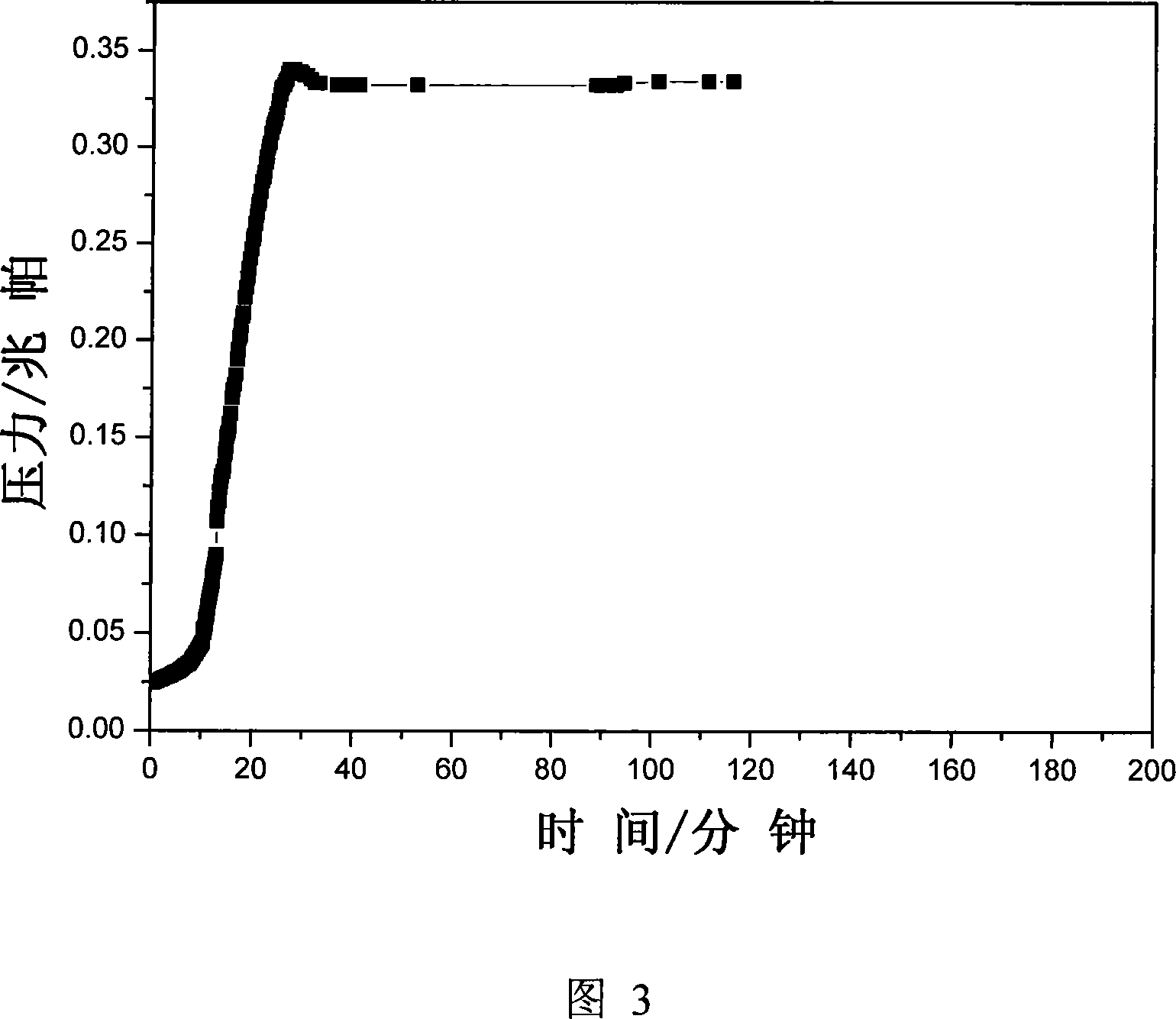
[0031] Pass 0.5MPa ammonia gas into the MgCl-filled 2 , CaCl 2 、CoCl 2 , CuCl 2 、NiCl 2 and MgCl 2 -CaCl 2 , CaCl 2 -Ca(NO 3 ) 2 , CuSO 4 -ZnSO 4 , MgCl 2 -CaCl 2 -CaSO 4 -ZnSO 4 stainless steel reactor, allowing it to react at room temperature to generate ammonia-saturated MgCl 2 -6NH 3 , CaCl 2 -8NH 3 、CoCl 2 -6NH 3 , CuCl 2 -8NH 3 、NiCl 2 -6NH 3 and (MgCl 2 -CaCl 2 )-14NH 3 , (CaCl 2 -Ca(NO 3 ) 2 )-16NH 3 , (CuSO 4 -ZnSO 4 )-16NH 3 and (MgCl 2 -CaCl 2 -CaSO 4 -ZnSO 4 )-36NH 3 , then take by weighing 5g of the gained sample and put it into a ball milling tank, the ball-to-material ratio is 40:1, and the ball milling time is 12h. In the ball milling tank, argon is filled as a protective gas, and mechanical ball milling is carried out to test the ammonia release performance of the sample after ball milling. The results are listed in Table 2. It can be seen from Table 2 that after ball milling, the sample can be rapidly deaminated in the r...
Embodiment 3
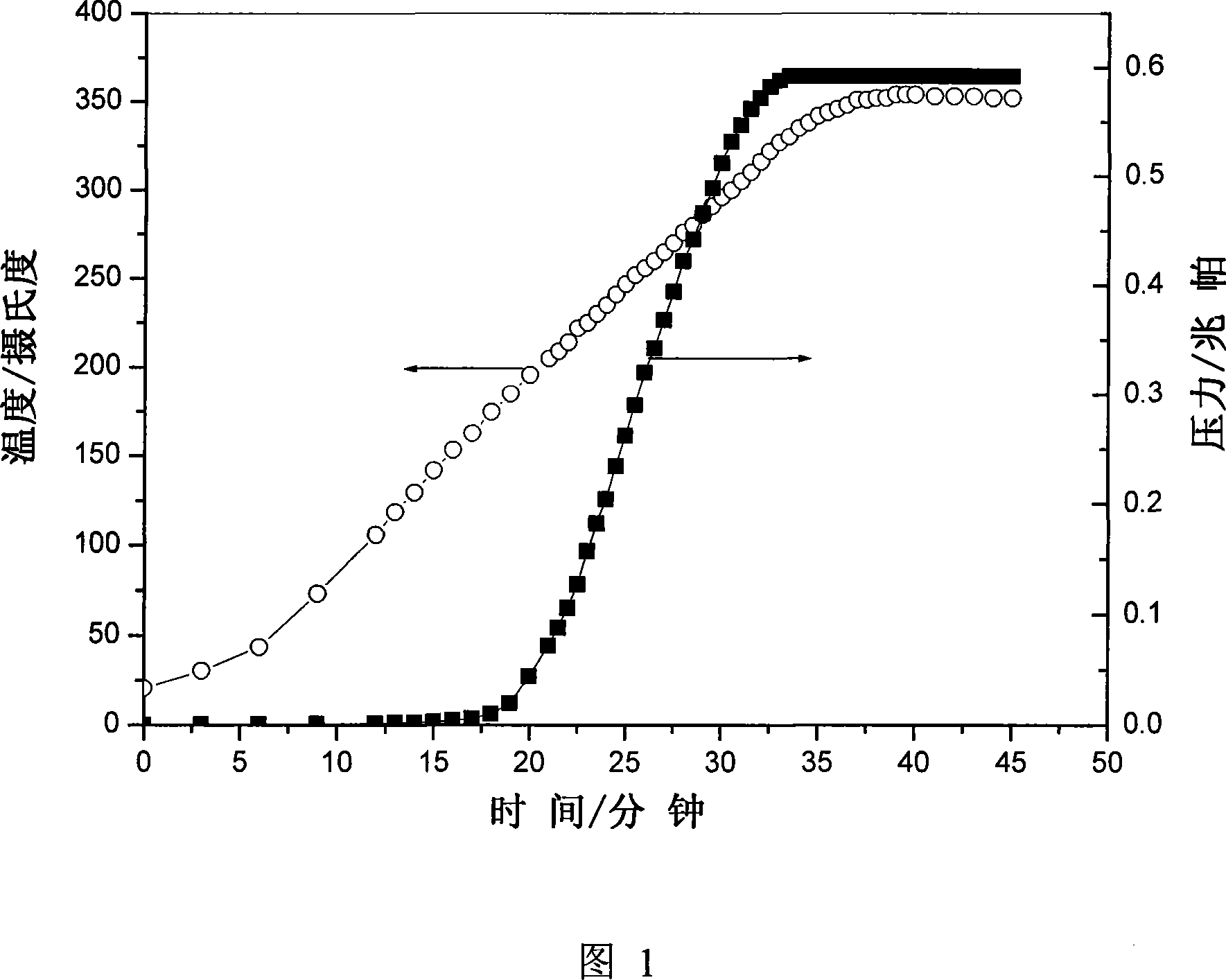
[0035] Anhydrous MgCl 2 React with 0.5MPa ammonia to generate ammonia-saturated amino complex MgCl 2 (NH 3 ) 6 , and a temperature-rising desorption experiment was performed on it. Figure 1 is Mg(NH 3 ) 6 Cl 2 The relationship between deamination rate and temperature at a heating rate of 10°C / min. It can be seen from the figure that saturated Mg(NH 3 ) 6 Cl 2 Ammonia is released rapidly at 160°C, and the release of ammonia is completed when the temperature reaches 330°C, the pressure of the released ammonia gas can reach ~0.6MPa, and the process of releasing ammonia is also completed within ~20min, showing that the material has excellent kinetics of releasing ammonia performance. The reaction equation of this process is Mg(NH 3 ) 6 Cl 2 (s)→MgCl 2 (s)+6NH 3 (g). When the temperature is lower than 100°C, the ammonia pressure in the system is lower than 0.001MPa. For the 5MPa low-pressure ammonia synthesis system, this shows that after the adsorption operation, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com