Preparation method of magnetic nanometer iron oxide adsorbent for removing arsenic from water
A magnetic nano, iron oxide technology, applied in chemical instruments and methods, adsorbed water/sewage treatment, alkali metal oxides/hydroxides, etc. complex problems, to achieve the effects of low raw material and preparation cost, short synthesis cycle, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
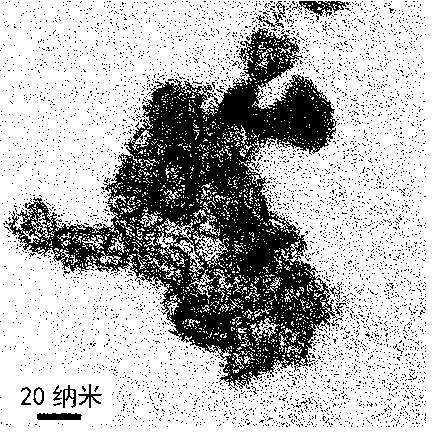
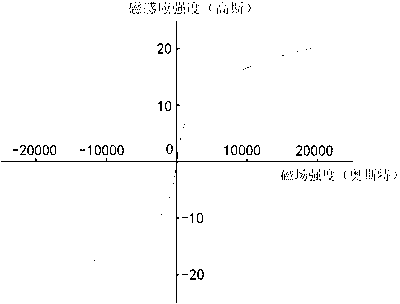
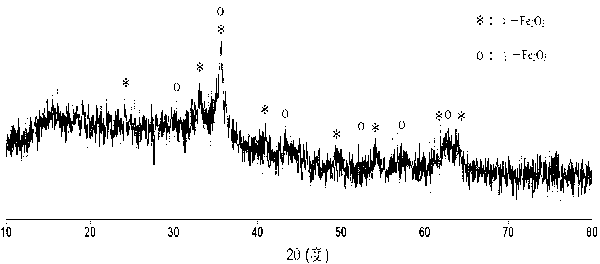
[0024] Dissolve 16.2 grams of ferric nitrate nonahydrate in 180 milliliters of pure water, dissolve 6.4 grams of sodium hydroxide in 100 milliliters of pure water, mix the above two solutions, centrifuge the precipitate, add 7.2 grams of glacial acetic acid (iron and glacial acetic acid The molar ratio is 1:3), magnetically stirred for about 1 hour until the hydrated iron oxide colloid was formed, then added 400 ml of acetone and continued to stir for 1 hour, after standing still, centrifuged, dried, and then roasted in the air at 250 ° C for 3 hours, That is to obtain magnetic nano-iron oxide, and the transmission electron microscope shows that the basic particle size is about 10-30 nanometers (such as figure 1 shown), the magnetization curve shows that it is magnetic in a magnetic field (as figure 2 shown), X-ray diffraction structure analysis shows that the main crystal phase of the material is α-Fe 2 o 3 with γ-Fe 2 o 3 (Such as image 3 shown). Each gram of the mat...
Embodiment 2
[0026] Dissolve 10.8 grams of ferric chloride hexahydrate in 180 milliliters of pure water, dissolve 8.5 grams of sodium carbonate in 100 milliliters of pure water, mix the above two solutions, centrifuge the precipitate, add 14.4 grams of glacial acetic acid (iron and glacial acetic acid The molar ratio is 1:6), magnetically stirred for about 1 hour until the hydrated iron oxide colloid was formed, then added 100 ml of propanol and continued to stir for 1 hour, after standing still, centrifuged, dried, and then roasted in nitrogen at 250 ° C for 3 hours , that is, the magnetic nano-iron oxide is obtained, and the X-ray diffraction structure analysis shows that the main crystal phase of the material is γ-Fe 2 o 3 (Such as Figure 4 shown). Each gram of the material can absorb more than 30 mg of inorganic trivalent arsenic and more than 40 mg of inorganic pentavalent arsenic in water.
Embodiment 3
[0028] Dissolve 16.2 grams of ferric nitrate nonahydrate in 180 milliliters of pure water, dissolve 6.4 grams of sodium hydroxide in 100 milliliters of pure water, mix the above two solutions, centrifuge the precipitate, add 120 grams of glacial acetic acid (iron and glacial acetic acid The molar ratio is 1:50), magnetically stirred for 0.3 hours until the hydrated iron oxide colloid was formed, then added 100 ml of propionaldehyde and continued to stir for 1 hour, left to stand, centrifuged, dried, and then baked in argon at 600 °C for 3 hours , that is, the magnetic nano-iron oxide is obtained, and the X-ray diffraction structure analysis shows that the main crystal phase of the material is γ-Fe 2 o 3 . Each gram of the material can absorb more than 20 mg of inorganic trivalent arsenic and more than 30 mg of inorganic pentavalent arsenic.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com