ª‡-N-acetyl galactosamine enzyme and encode gene and applications thereof
A technology that encodes genes and genes, which is applied in the fields of application, genetic engineering, and plant genetic improvement, etc., and can solve problems such as incomplete enzymatic hydrolysis and inability to meet the requirements of blood type transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, cloning of α-N-acetylgalactosaminidase α NAGA coding gene
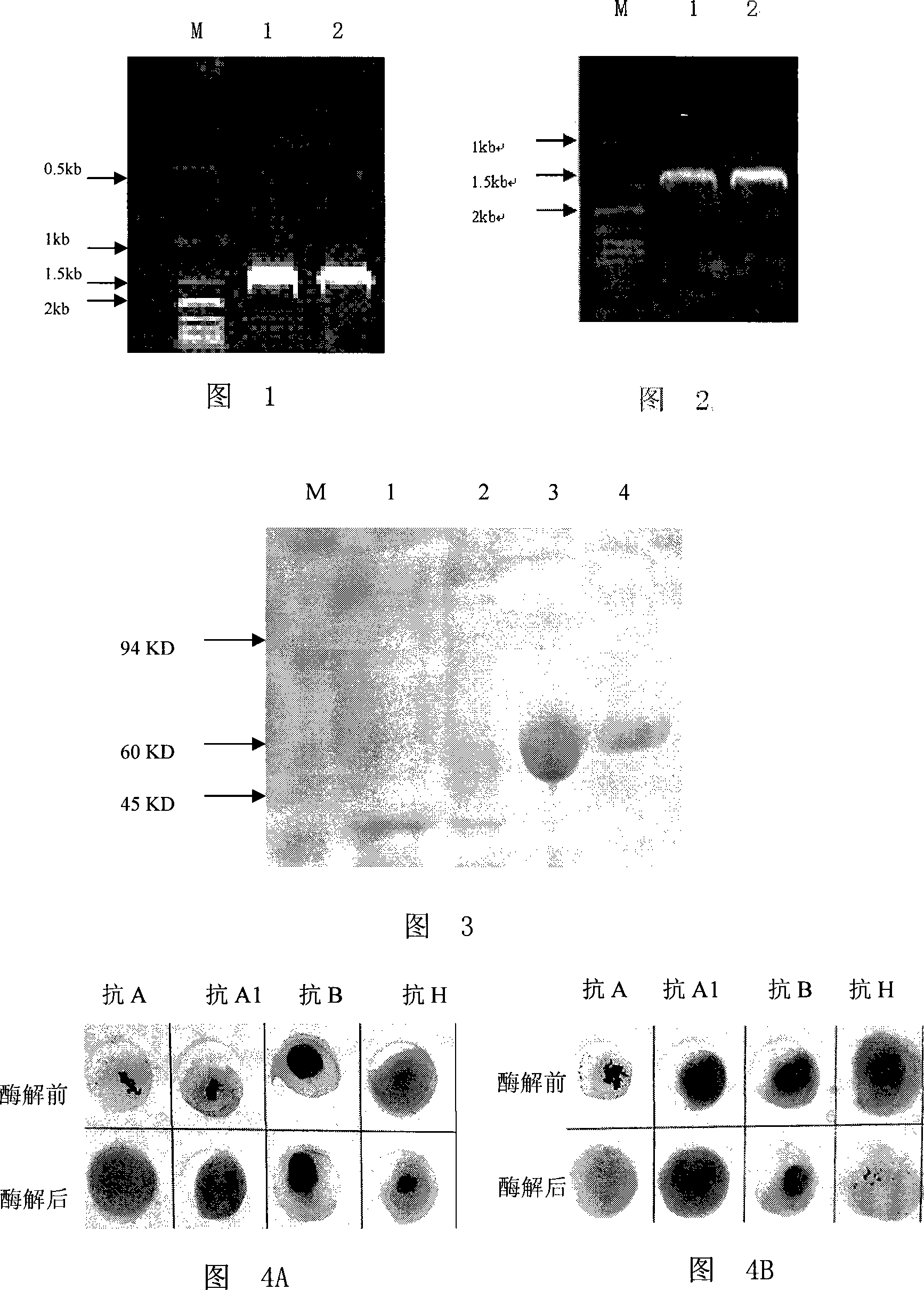
[0049] Inoculate the Chryseobacterium meningosepticum (Chryseobacterium meningosepticum) ZYP01 CGMCCNo.2198 strain into the culture medium (5g / L yeast extract, 5g / L peptone, 5g / L NaCl, 3g / L K2HPO4, the rest is water. pH7.2) , 200rpm, and cultivate overnight at 30°C. Genomic DNA was extracted according to the instructions of the Promega Genomic DNA Extraction Kit. Using the extracted genomic DNA as a template, PCR was performed using an upstream primer (sequence: 5'-ATGGGCGCCTTAATTCCC-3') and a downstream primer (sequence: 5'-TTAGTAGTCGTCATTTATTGC-3'). The PCR conditions were as follows: pre-denaturation at 95°C for 5 min; then denaturation at 95°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 90 s, a total of 30 cycles; finally, extension at 72°C for 10 min. The PCR product was detected by agarose gel electrophoresis, and a band with a molecular weight of about 1335 bp was obta...
Embodiment 2
[0051] Embodiment 2, the expression of αNAGA
[0052] 1. Construction of the expression vector pET-22b-αNAGA containing the gene encoding αNAGA
[0053] Using pTE-αNAGA as a template, with an upstream primer (sequence: 5'-ATCATATGCCAAAAAAGTAAGAATTGC-3' (with Nde I restriction site)), and a downstream primer (sequence: 5'-TGCTCGAGGTAGTCGTCATTTATTGC-3' (with Xho I Restriction site) PCR amplified nucleotide sequence is the αNAGA gene fragment from the 52-1332th deoxyribonucleotide at the 5' end of sequence 1 in the sequence listing. PCR conditions are: first 95 ℃ pre-denatured for 5min; Denaturation at 95°C for 30s, annealing at 43°C for 30s, extension at 72°C for 90s, a total of 30 cycles; final extension at 72°C for 10min. The PCR product was detected by agarose gel electrophoresis, and a band with a molecular weight of about 1300bp was obtained (in Figure 1, Lane 2).
[0054] According to the operation guidelines of TAKARA company, the PCR product was connected to the pMD-18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com