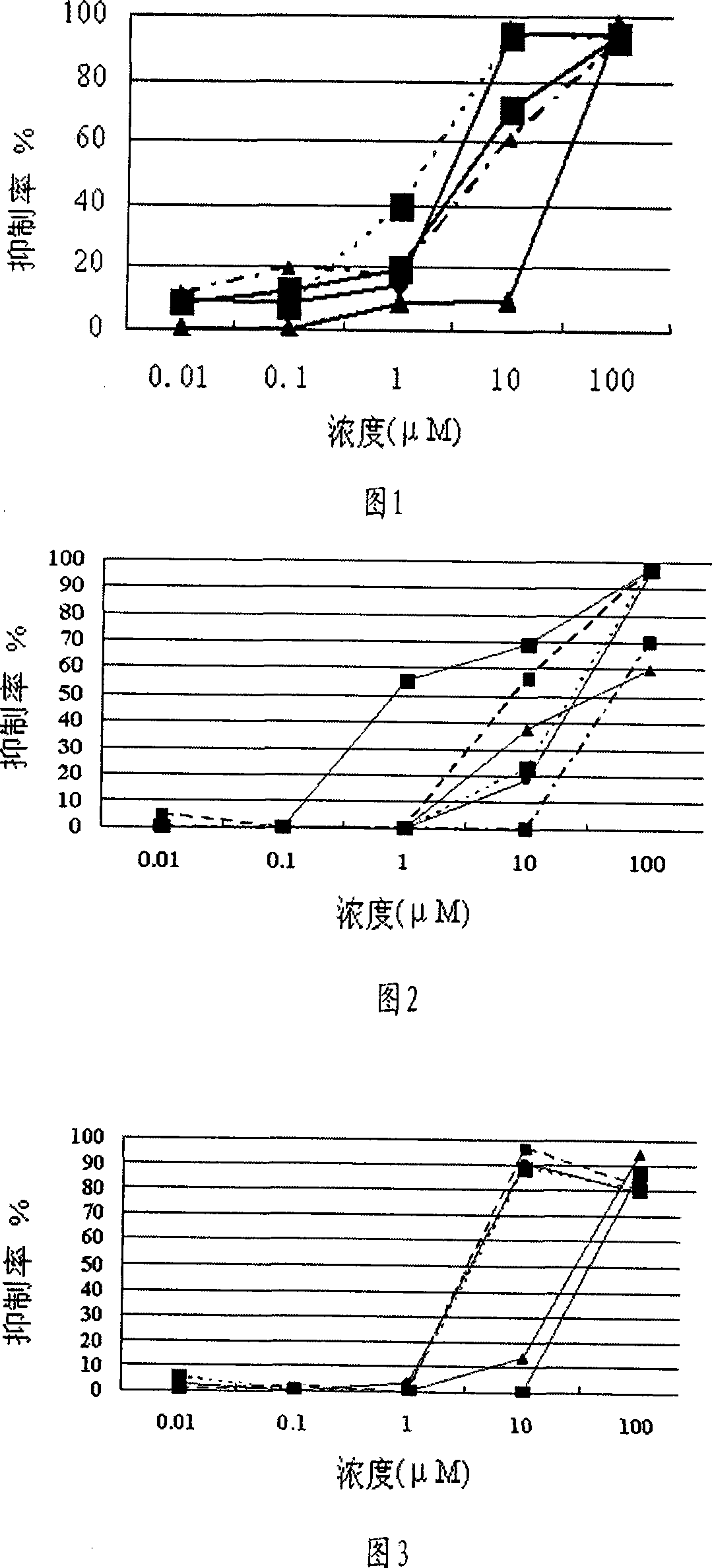
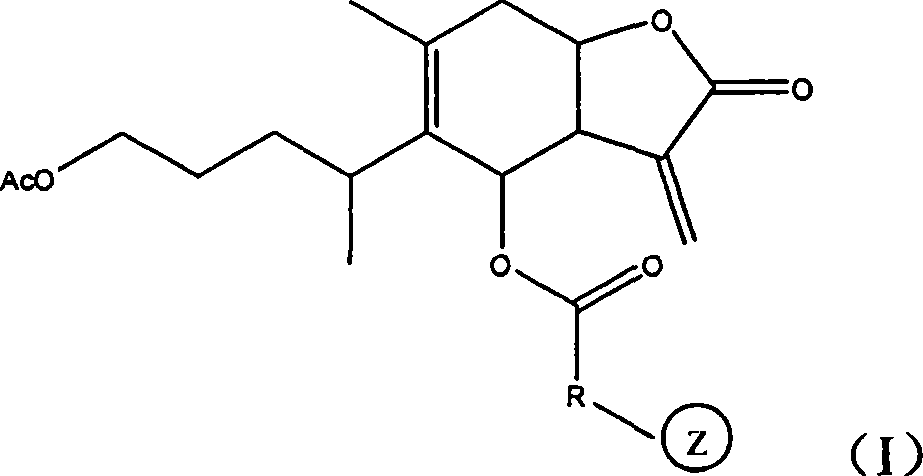
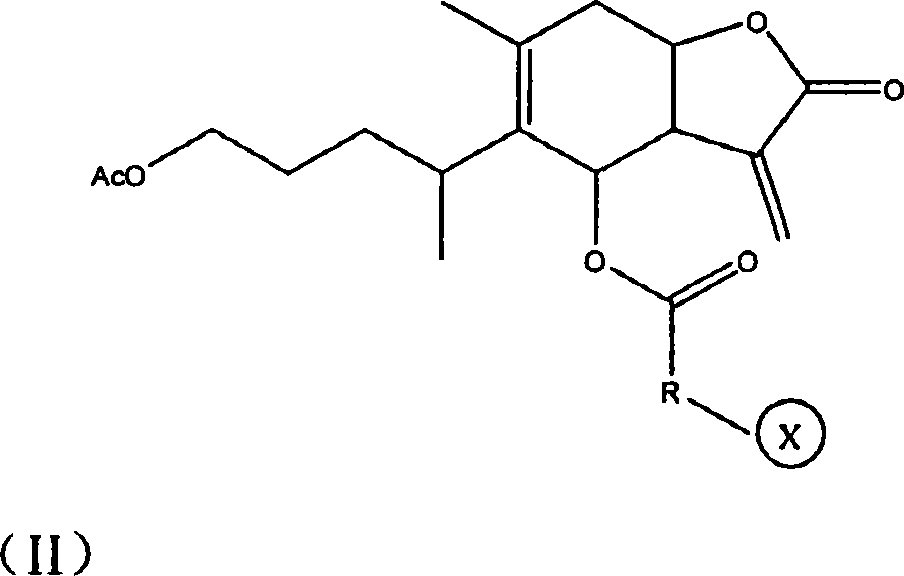
1-oxy-acetyl inula britannica lactone derivative, preparation method thereof and application of the same in preparing cancer-curing medicine
A technology of Inula grandiflora and Inula grandiflora, applied in drug combination, antineoplastic drugs, organic chemistry, etc., can solve the problems of weak pharmacological activity and inability to meet clinical needs, and achieve significant anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] One of the methods for preparing compound (V)
[0092] With 1-O-ABL 113.2mg (0.4mmol), 2-(4-isobutylphenyl) propionic acid 85.7mg (0.4mmol) DMAP (4-dimethylaminopyridine) 58.6mg (0.4mmol) DCC99.3mg (0.45mmol) was dissolved in 3.5ml of dichloromethane and stirred at room temperature for 24h. After the reaction solution was diluted with 20ml of dichloromethane, it was washed with 5% HCl (20ml), saturated NaHCO3 solution (15ml×2), and then with saturated NaCl solution (15ml) until neutral, and dried overnight with anhydrous MgSO4. After filtration, dichloromethane was distilled off under reduced pressure to obtain a white solid. Separation by silica gel G column chromatography [petroleum ether-ethyl acetate (3:1)], the oily substance 64(S)-2-(4-isobutylphenyl)propionyl]-1-O-ABL (36mg ) yield 23% and 6-[(R)-2-(4-isobutylphenyl)propionyl]-1-O-ABL (78 mg) yield 45%.
[0093] The compound has the following physical properties:
[0094] colorless oil;
[0095] Spectral data:...
Embodiment 2
[0104] The second method of preparing compound (V)
[0105] (1) Preparation of 2-(4-isobutylphenyl) propionyl chloride
[0106] Take 500mg (2.4mmol) of 2-(4-isobutylphenyl)propionic acid, add 2ml of thionyl chloride, heat and reflux until no HCl gas overflows, after the reaction is complete, remove excess thionyl chloride by distillation under reduced pressure, add Dilute with 5ml of dichloromethane and set aside.
[0107] (2) Dissolve 100mg (0.37mmol) of 1-O-ABL and 50mg (0.4mmol) of DMAP in 5ml of dichloromethane, cool in an ice bath to 0°C, stir and add the above acid chloride dropwise, after 20min, remove the ice after 1h of reaction After the reaction is completed, the reaction solution is diluted with 20ml of dichloromethane, washed with 5% HCl (30ml), saturated NaHCO3 solution (30ml × 2), and then washed with saturated NaCl solution (30ml) to medium properties, dried over anhydrous MgSO4 overnight. After filtering, the dichloromethane was distilled off under reduced ...
Embodiment 3
[0110] Preparation of compound (VI)
[0111] Take 1-O-ABL and phenylacetic acid for condensation reaction. The specific reaction conditions are the same as in Example 1 to generate 6-(2-phenylacetoxy)-1-O-ABL. The yield was 41%.
[0112] The compound has the following physical properties:
[0113] Colorless oil.
[0114] Spectral data:
[0115] 1 HNMR (CDCl 3 , ppm): 0.96 (d, 3, 15-CH 3 ), 1.03(m, 1, H-3a), 1.27(m, 2, H-3b, H-2a), 1.41(m, 1, H-2a), 1.80(s, 3, 14-CH 3 ), 2.05 (s, 3, COCH 3 ), 2.50(dd, H, H-9a), 2.74(dd, 2, H-9b, H-4), 3.52(dd, 1, H-7), 3.93(m, 2, H-1), 4.94(t, 1, H-8), 5.3(s, 1, H-6), 5.45(d, 1, H-13a), 6.35(d, 1, H-13b), 3.6(d, 2, Ar-C H 2 ), 7.2 (dd, 2, Ar -CH 2 ), 7.34 (dd, 2, Ar -CH 2 ), 7.26 (dd, 1, pH -CH 2 ).
[0116] Elemental analysis: calculated to contain C, 70.40; H, 7.09; O, 22.51; measured to contain C, 70.43; H, 7.10; O, 22.47
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com