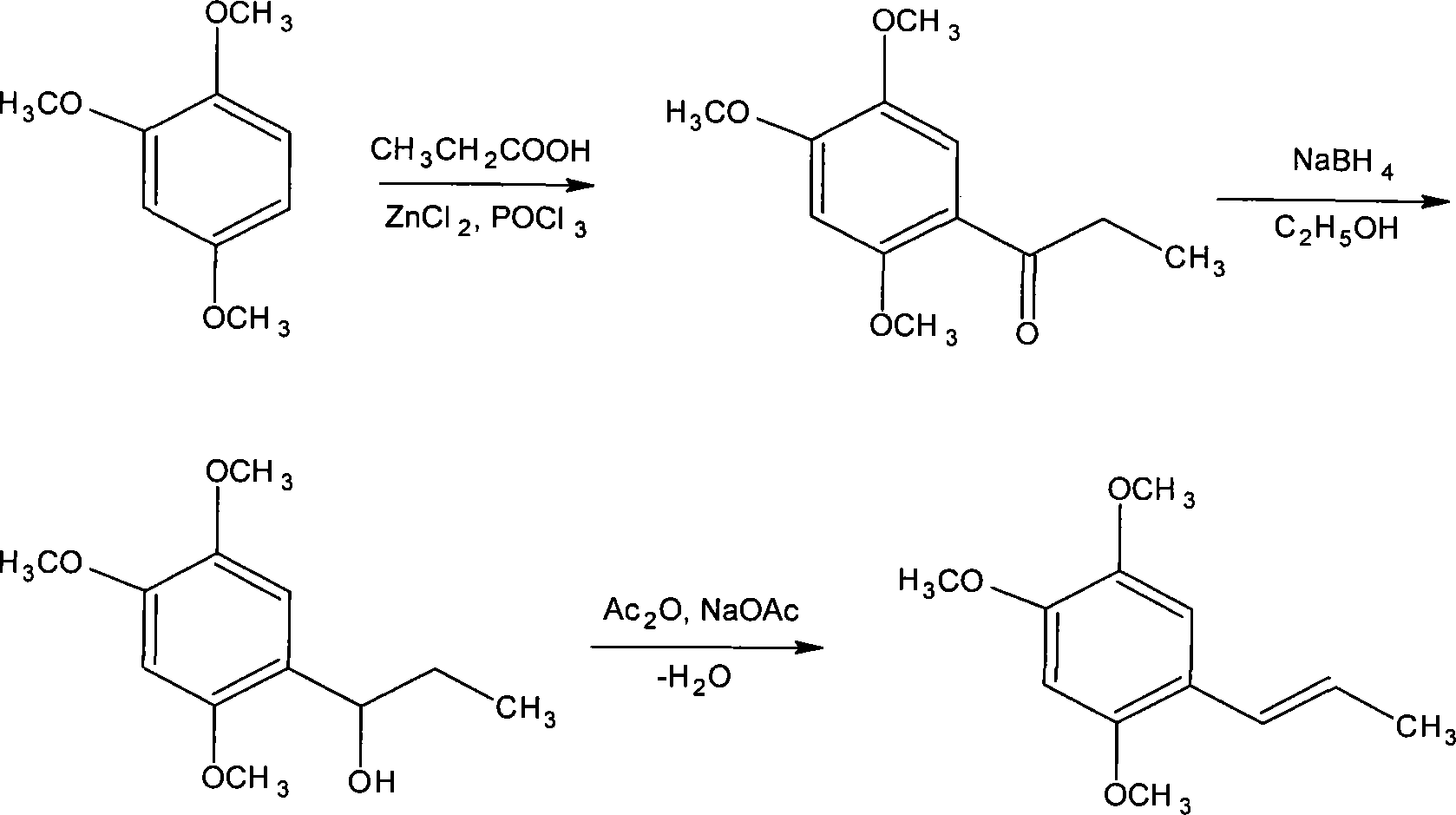
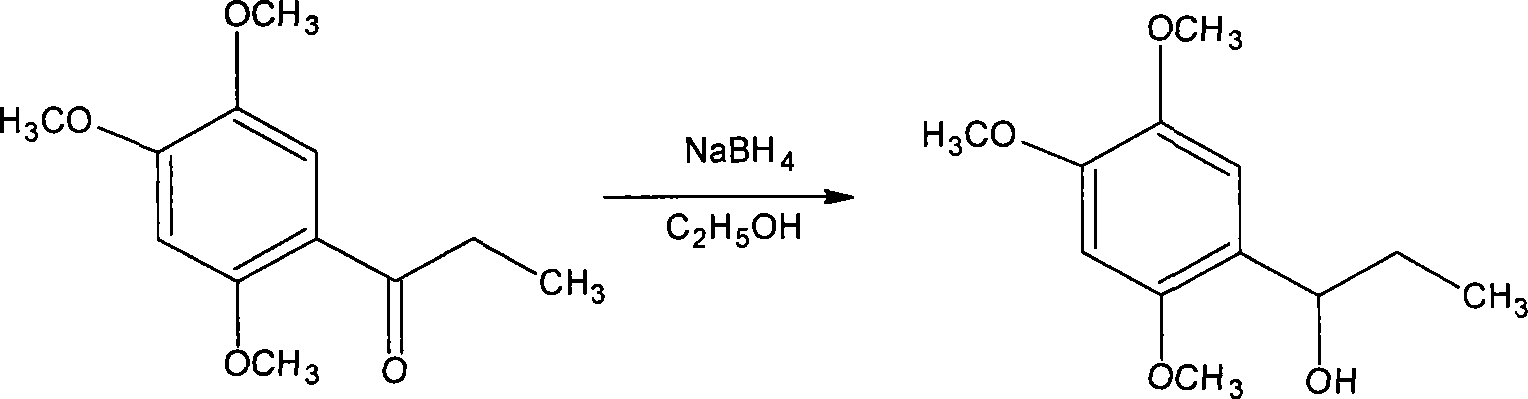
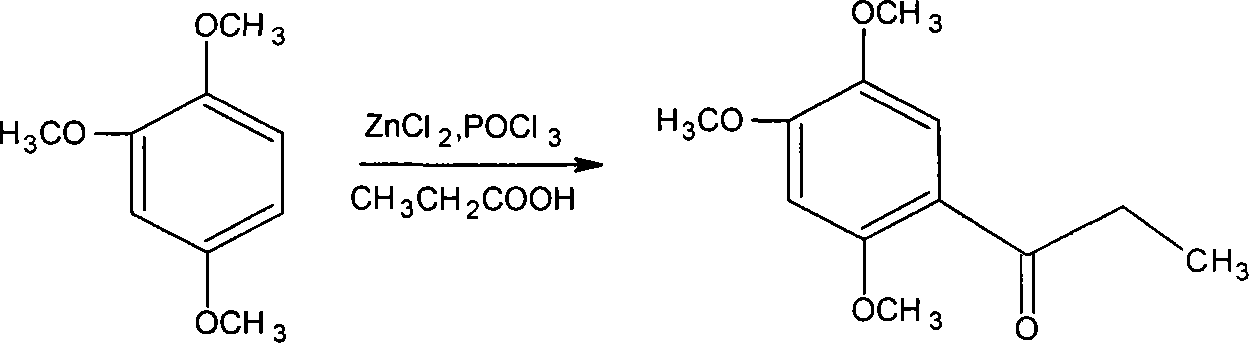Novel method for synthesizing alpha-asarone
A technology of asarone and trimethoxybenzene, which is applied in the new synthesis field, can solve the problems of cumbersome operation, high cost, and low yield of α-asarone, and achieve the effect of simple operation and improved production yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Add 4 grams of anhydrous zinc chloride to the dry reaction flask, add 30 ml of phosphorus oxychloride, and stir the reaction at 50°C, then add 1.04 grams of propionic acid and 1.68 grams of 1,2,4-trimethoxybenzene, and stir the reaction After 1 to 5 hours, the end point of the reaction was detected by TLC, cooled with ice water, and stirred until solids were precipitated. Filter, wash with water until neutral, and dry to obtain 2 g of off-white small needle crystal product. The yield was 89.29% (based on 1,2,4-trimethoxybenzene), and the product was recrystallized from methanol for use. m.p106-108°C.
Embodiment 2
[0057] Add 10 grams of anhydrous zinc chloride (or anhydrous aluminum trichloride) in the dry reactor, add 25 milliliters of phosphorus oxychloride and sodium polyphosphate (or monopotassium hydrogen phosphate or potassium dihydrogen phosphate), Stir the reaction at 30°C, then add 2.3 grams of propionic acid and 3.36 grams of 1,2,4-trimethoxybenzene, stir the reaction for 1 to 5 hours, check the end point of the reaction with TLC, cool with ice water, and stir until the solid precipitates. Filter, wash with water until neutral, and dry to obtain 3.9 g of an off-white product with a yield of 87.05%. Recrystallize with methanol to obtain a small needle crystal product, which is dried for use.
Embodiment 3
[0059] Add 15 grams of anhydrous zinc chloride to the dry reaction bottle, add 40 ml of phosphorus oxychloride (or phosphoric acid), and an appropriate amount of potassium dihydrogen phosphate or dipotassium hydrogen phosphate or potassium phosphate, stir the reaction at 70 ° C, and then add 3.7 grams of propionic acid and 5.04 grams of 1,2,4-trimethoxybenzene were stirred and reacted for 1 to 5 hours, and the end point of the reaction was detected by TLC, cooled with ice water, and stirred until solids were precipitated. Filter, wash with water until neutral, and dry to obtain 5.8 g of small needle crystal product with a yield of 86.31%. The product is recrystallized with methanol and dried for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com