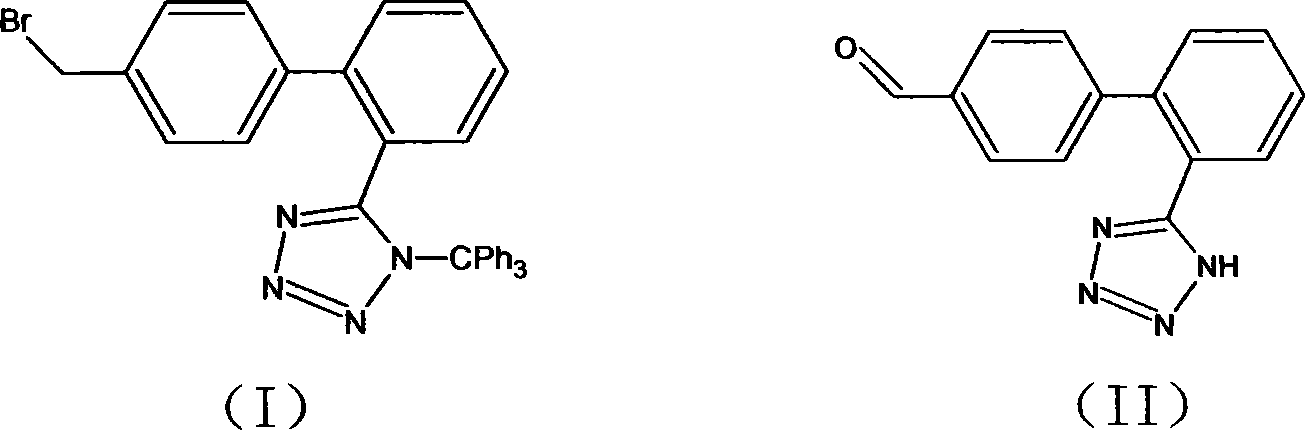
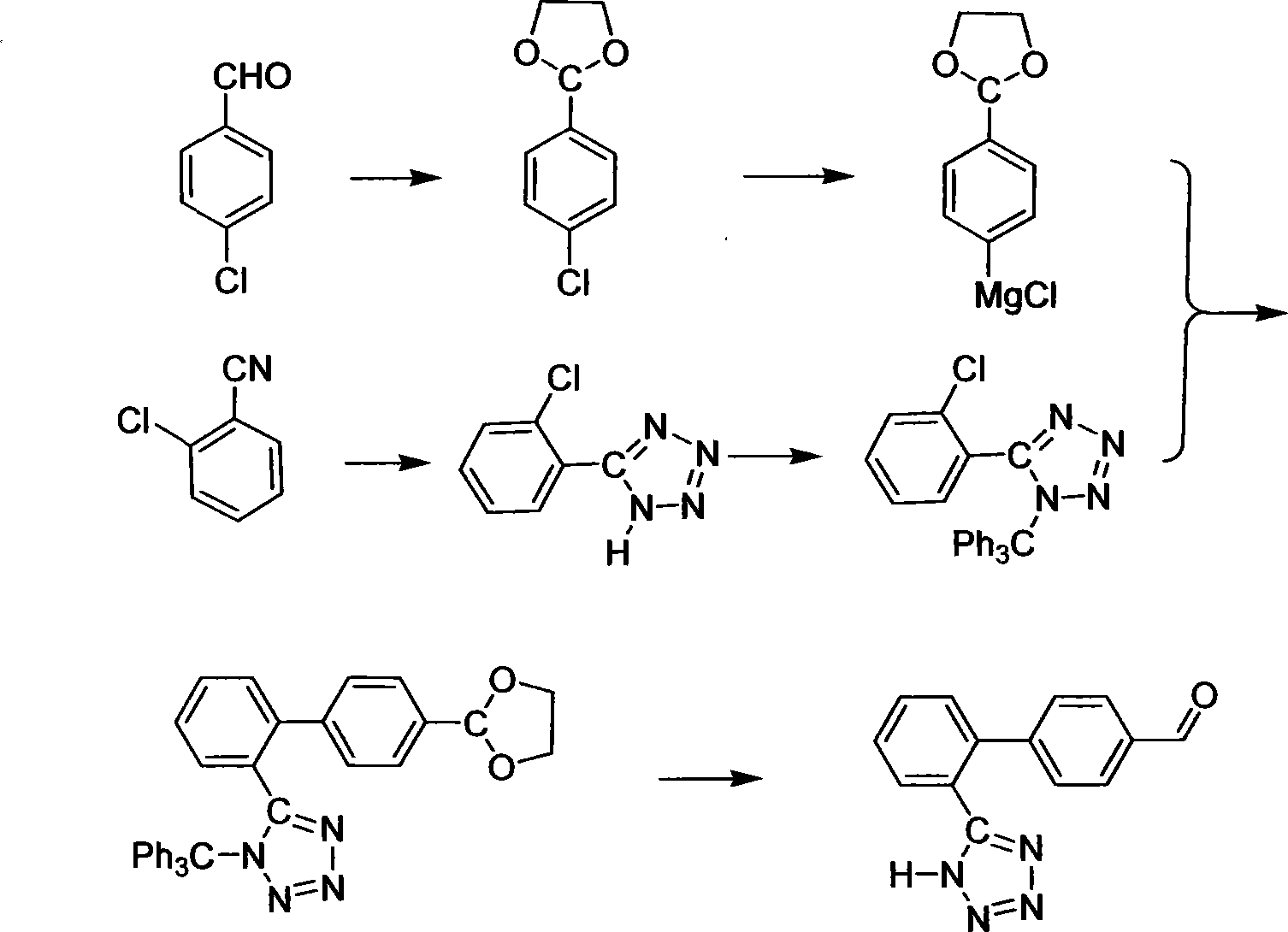
Method for preparing sartan drug main ring 5-(4'-formyl biphenyl-2-group)-1H-tetrazole treating hypertension
A formyl biphenyl and high blood pressure technology, applied in the chemical pharmaceutical field, can solve problems affecting product purity and yield, unfavorable production and labor protection, flammable and explosive, etc., to achieve suppression of side reactions, reduction of emissions, and discharge of three wastes little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
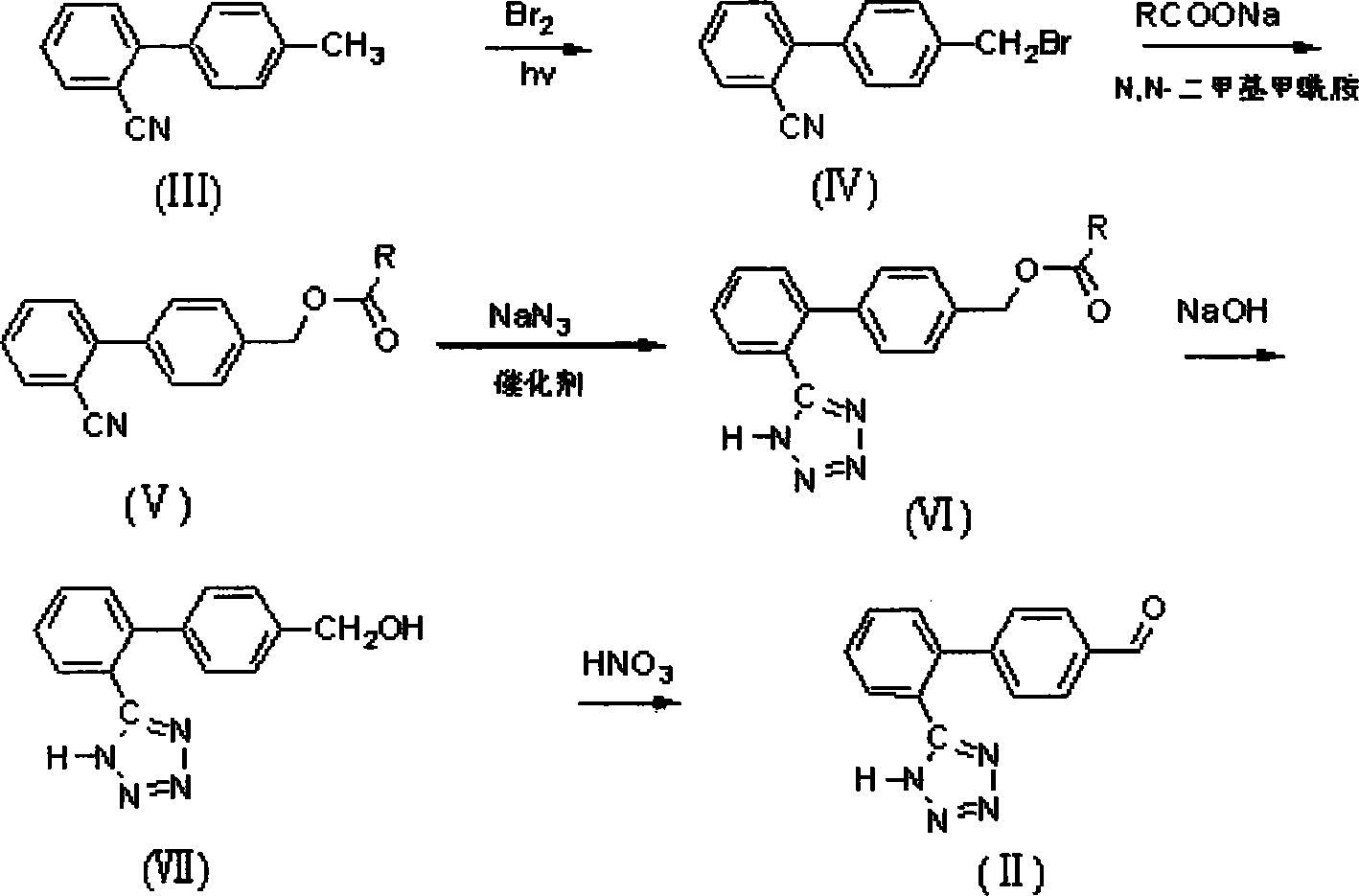
[0029] Bromination Reaction: Preparation of Sartan Bromide IV
[0030] In the flask, add 19.3g (0.10mol) of sartan biphenyl III and 200ml of chloroform, under stirring, heat up to 40°C, turn on the high-pressure sodium lamp, and slowly add about 16.0g (0.10mol) of bromine (sartan biphenyl The molar ratio of III and bromine=1:1), control the rate of addition, the reaction produces a large amount of hydrogen bromide gas, which is absorbed with liquid caustic soda, and the bromine is added dropwise in about 1.5 hours. After 4.5 hours of reaction, the chloroform was distilled off, and 100 ml of absolute ethanol was added for recrystallization; after drying, 24.0 g of a white solid product was obtained, the HPLC analysis content was 98%, and the yield was 88.2%;
[0031] Esterification reaction: Preparation of sartan benzyl ester V
[0032] In the flask, add 27.1g (0.10mol) sartan bromide IV, 10.7g (0.13mol) sodium acetate (the molar ratio of sartan bromide IV to sodium acetate=1:...
Embodiment 2
[0040] Bromination Reaction: Preparation of Sartan Bromide IV
[0041] In the flask, add 19.3g (0.10mol) of sartanbiphenyl III and 200ml of chloroform, under stirring, heat up to 61°C for reflux reaction, turn on the high-pressure sodium lamp, and slowly add about 19.2g (0.12mol) of bromine (sartanbiphenyl) dropwise. The molar ratio of benzene III to bromine=1:1.2), control the rate of addition, so that the liquid under the reflux condensation has no obvious yellow, the reaction produces a large amount of hydrogen bromide gas, which is absorbed with liquid caustic soda, and the bromine is added dropwise in about 2 hours. After the addition, continue to keep warm and light for 3 hours, and the reaction is completed for a total of 5 hours; then evaporate the chloroform, add 100ml of absolute ethanol for recrystallization; after drying, 24.4g of a white solid product is obtained, the HPLC analysis content is 99%, and the yield is 90%;
[0042] Esterification reaction: Preparation...
Embodiment 3
[0051] Bromination Reaction: Preparation of Sartan Bromide IV
[0052] In the flask, add 19.3g (0.10mol) of sartan biphenyl III and 200ml of dichloroethane, under stirring, raise the temperature to 70°C for reaction, turn on the high-pressure sodium lamp and slowly add about 16.0g (0.10mol) of bromine (sartan The molar ratio of tanbiphenyl III to bromine=1:1), control the rate of addition, the reaction produces a large amount of hydrogen bromide gas, which is absorbed with liquid caustic soda, and the bromine is added dropwise in about 1.5 hours. Hours, a total of 4.5h to complete the reaction, dichloroethane was distilled off, and 100ml of absolute ethanol was added for recrystallization; after drying, 24.2g of a white solid product was obtained, the HPLC analysis content was 98%, and the yield was about 89%;
[0053] Esterification reaction: Preparation of sartan benzyl ester V
[0054] In the flask, add 27.1g (0.10mol) sartan bromide IV, 10.7g (0.20mol) sodium acetate (the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com