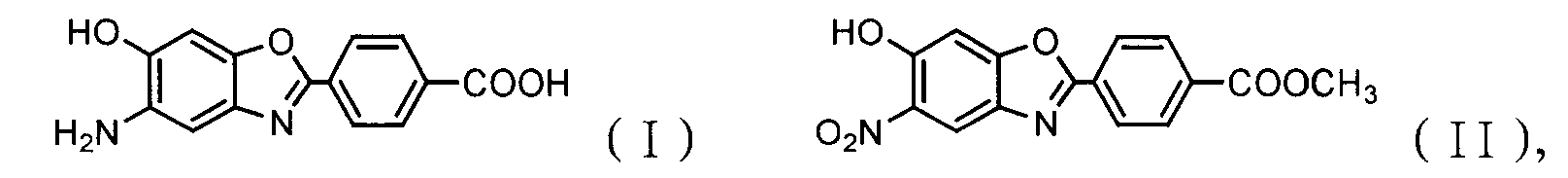Method for synthesizing 5-amide-6-hydroxy-2-(4-carboxylphenyl)benzoxazole
A p-carboxyphenyl, synthesis method technology, applied in the field of synthesis of 5-amino-6-hydroxy-2-benzoxazole, can solve problems hindering the preparation, expansion and application of high-performance PBO materials, and achieve good industrialization Prospects and advantages, reduce production costs, and produce obvious advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation of 5-amino-6-hydroxyl-2-(p-carboxyphenyl) benzoxazole (ABA)
[0034] 4.0g K 2 CO 3 Dissolve in 150ml of water solvent, add 3.0g NHAB (purity 96.5%) into the dilute alkali solution, stir and heat up to 80°C, hydrolyze for 50min until the solution is clear, and slowly add 8.0 g Na 2 S 2 o 4 , control the reduction reaction at 0-5°C for 20 minutes, filter to obtain yellow crystals, wash with ice water, and vacuum-dry to obtain 2.12 g of light yellow crystalline products with a purity of 98.9%. After qualitative FT-IR, 13 C-NMR, 1 H-NMR attribute analysis and elemental analysis, determined to be a new monomer of acid type AB type PBO: 5-amino-6-hydroxyl-2-(p-carboxyphenyl)benzoxazole ABA monomer, yield 84.2% .
[0035] FT-IR (KBr, cm -1 ): 3333.4, 3269.7, 3151.1, 1676.8, 1617.0, 1579.4, 1466.6, 1409.7, 1380.8, 1294.0, 1177.3, 1129.1, 1053.9, 972.9, 879.4, 860.1, 784.9, 709.7. Elemental analysis values: C, 62.03; H, 3.75; N, 10.45. Calcula...
Embodiment 2~6
[0037] Adopt the same operation of embodiment 1, get different parameters (different temperature, raw material purity and Na 2 S 2 o 4 Consumption) reacts, and the results are shown in Table 1:
[0038] Table 1 Hydrolysis of Na by NHAB 2 S 2 o 4 Reduction "one-pot method" to prepare ABA monomer
[0039]
Embodiment 7
[0040] Embodiment 7: Alkaline hydrolysis, hydrogenation reduction in-situ synthesis method prepares ABA
[0041] 4.5g K 2 CO 3 Dissolve in 180ml of water solvent, add 3.00g of NHAB (purity 94.4%) into the dilute alkali solution, stir and heat up to 60°C, hydrolyze for 60min until the solution is clear, transfer the reaction solution to a high-pressure reactor, add 0.3g 5% Pd / C, stirred vigorously at 30°C and 0.3-0.4MPa hydrogen pressure for 3 hours, filtered to remove the catalyst, cooled the filtrate to room temperature and decolorized it with activated carbon, then used NaHSO 3 The saturated aqueous solution was adjusted to pH 6.5-7.0 to precipitate a precipitate, which was filtered and vacuum-dried to obtain 2.05 g of a khaki crystalline product with a purity of 98.2% and a yield of 82.6%. FT-IR, 13 C-NMR, 1 H-NMR attribute analysis is the same as that of Example 1 and is characterized as ABA monomer.
[0042] FT-IR (KBr, cm -1 ): 3334.3, 3269.7, 3146.3, 1675.8, 1617....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com