Antimicrobial compositions for inhibiting growth and proliferation of a microbial biofilm on medical devices
一种医疗装置、生物薄膜的技术,应用在抗微生物组合物领域,能够解决延长抗微生物效应、药物不受控制、不足等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
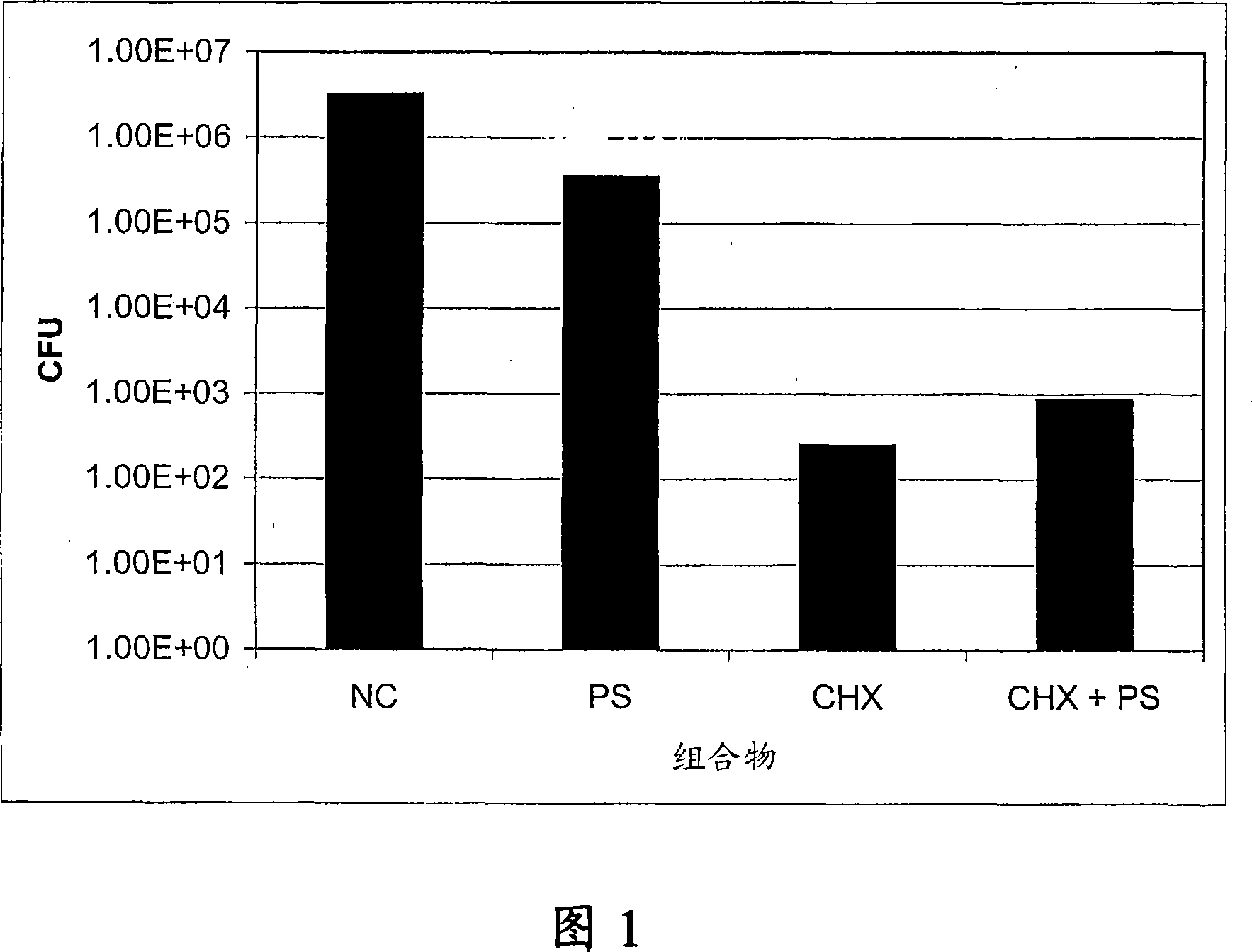
[0079] The binding effect of embodiment 1-protamine sulfate (PS) and chlorhexidine salt (CHX) Enhanced effect of film-embedded catheter-associated bacteria
[0080] An in vitro microplate assay (microplate assay) was performed to determine the effect of binding of protamine sulfate and chlorhexidine salt on biofilm formation of biofilm-embedded catheter-associated bacteria such as Escherichia coli, Pseudomonas aeruginosa Bacteria and the enhanced growth of Staphylococcus epidermidis. Overnight cultures of each strain grown in Luria-Bertani (LB) or Tryptic Soy Broth (TSB) were used as inoculum. In 12-well microplates, bacteria were grown on colony-forming antigens in the absence and presence (12.5, 25, or 50 μg / ml) of each test compound (PS or CHX), respectively, and together (PS+CHX). (CFA) medium (for Gram-negative) or in TSB (for Gram-positive). Plates were incubated at 37°C for 24 hours. The medium (media) containing planktonic cells in each well was gently removed an...
Embodiment 2
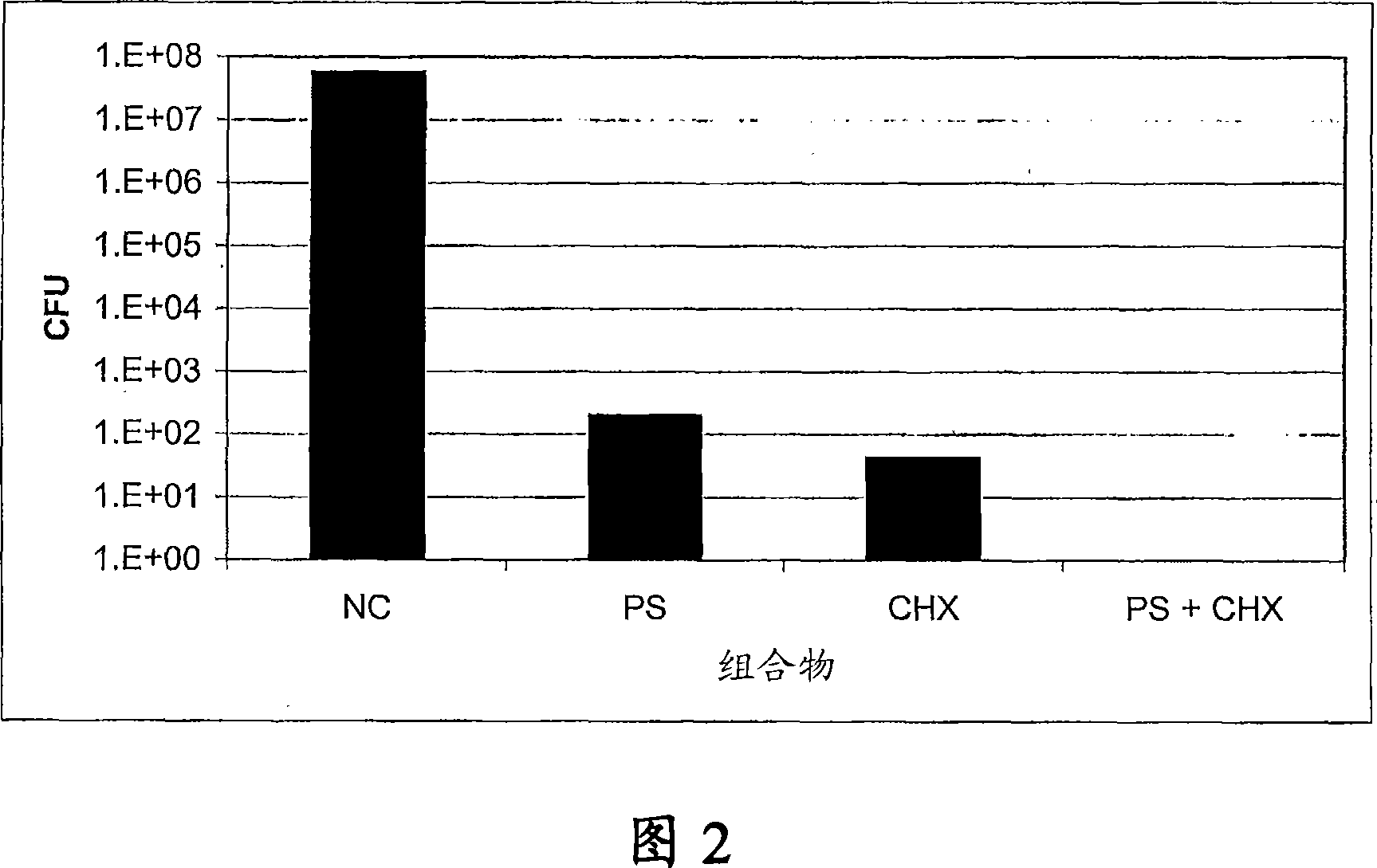
[0081] Example 2 - Protamine sulfate (PS) and chlorhexidine salt (CHX) combined with coated silicon Inhibitory activity of ketone catheters relative to catheter-associated bacteria
[0082] Antimicrobial activity of PS+CHX coated and uncoated 1 cm silicone catheter sections was assessed using the Kirby-Bauer technique as previously described by Sheretz et al. (Antimicrob. Agents. Chemother. 33: 1174-1178, 1989) . Catheters were coated by immersion in a PS (100 mg / ml) + CHX (400 mg / ml) solution followed by drying (as described in US Patent No. 6,475,434). Gas sterilize the catheter with ethylene oxide. Catheter-associated microorganisms such as Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, vancomycin-resistant enterococci (VRE), Staphylococcus epidermidis, Staphylococcus aureus, and albicans, grown in nutrient broth for 18 hours at 37°C. Appropriate inoculum of each strain or yeast strain was used to prepare sp...
Embodiment 3
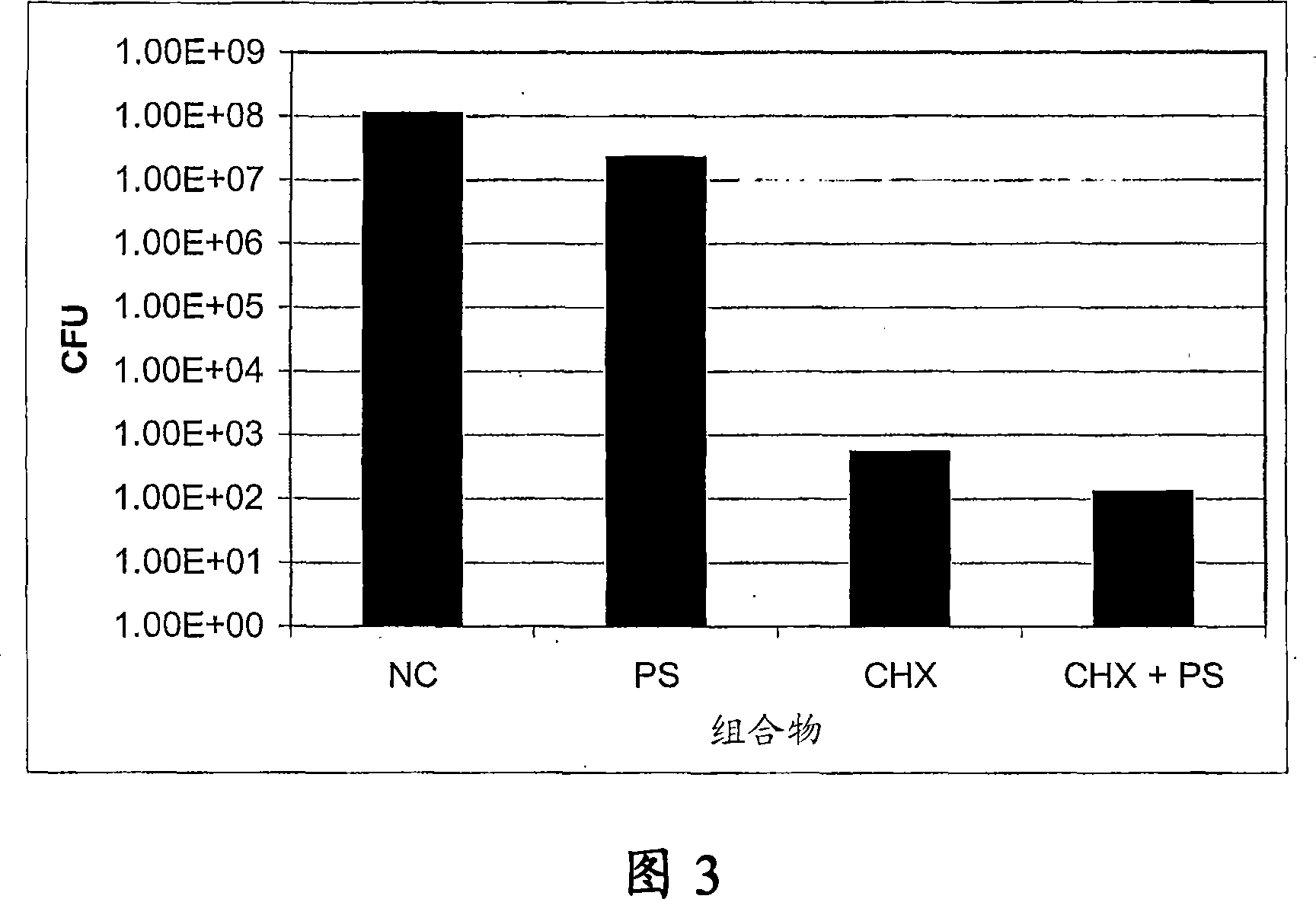
[0085] Example 3 - Protamine sulfate (PS) and chlorhexidine salt (CHX) combined with coated silicon Anti-adhesive effect of ketone catheters on catheter-associated bacteria
[0086] PS+CHX, PS, and CHX coated silicone catheters were tested for activity against bacterial colonization by exposing uncoated and coated sections in triplicate to E. coli, Pseudomonas aeruginosa, and Staphylococcus epidermidis . Silicone catheters were coated with PS (100 mg / ml), CHX (100 mg / ml) and PS (100 mg / ml)+CHX (100 mg / ml) and then gas sterilized with ethylene oxide. The coated catheter sections were incubated in sterile artificial urine at 100 rpm at 37°C for 24 hours prior to challenge with bacteria. After incubation, the catheter section was rinsed with sterile water and incubated in bacterial culture (in BHI medium) at 37°C for 3 hours at 100 rpm. After 3 hours of incubation, the sections were washed gently twice. Each washed section was transferred to a sterile tube containing 1 ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com