Method and apparatus for detecting analytes using an acoustic device
An analyte and device technology, which is applied in the field of detecting one or more analytes in fluid samples, can solve the problems of energy consumption, heating conductive fluid, and destroying biological analytes, so as to improve accuracy and sensitivity, and combine strong Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0165] Example I: General method for functionalizing the surface of flexible wave devices with capture agents
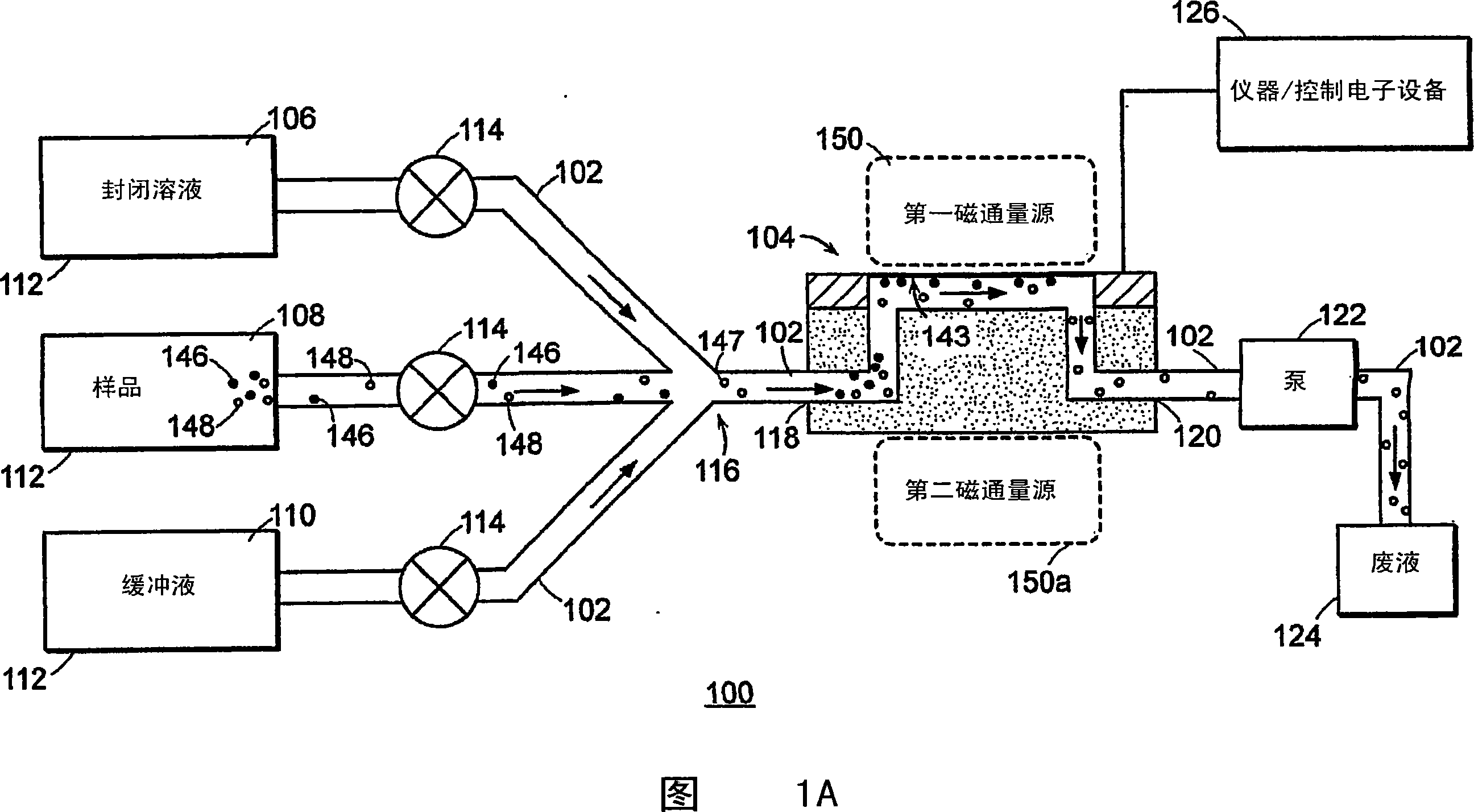
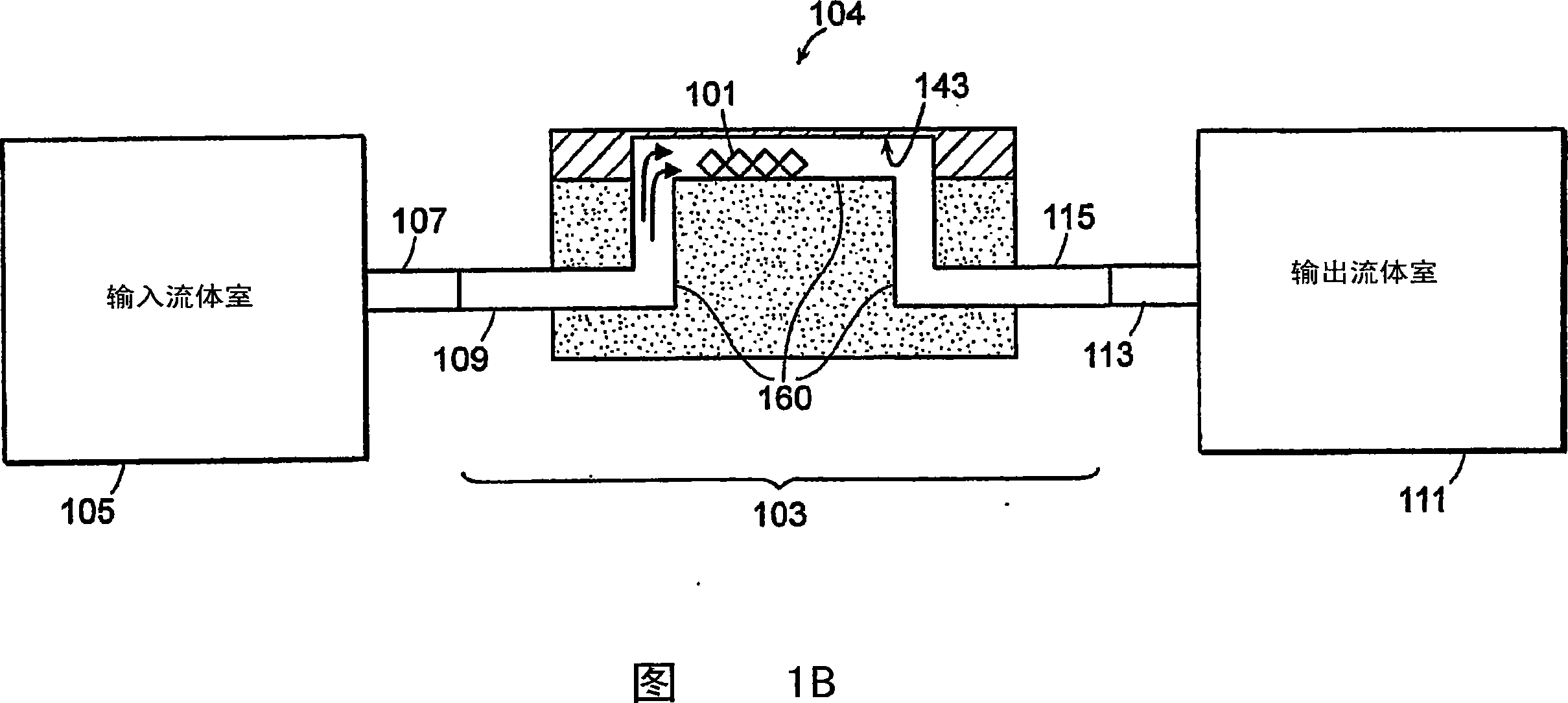
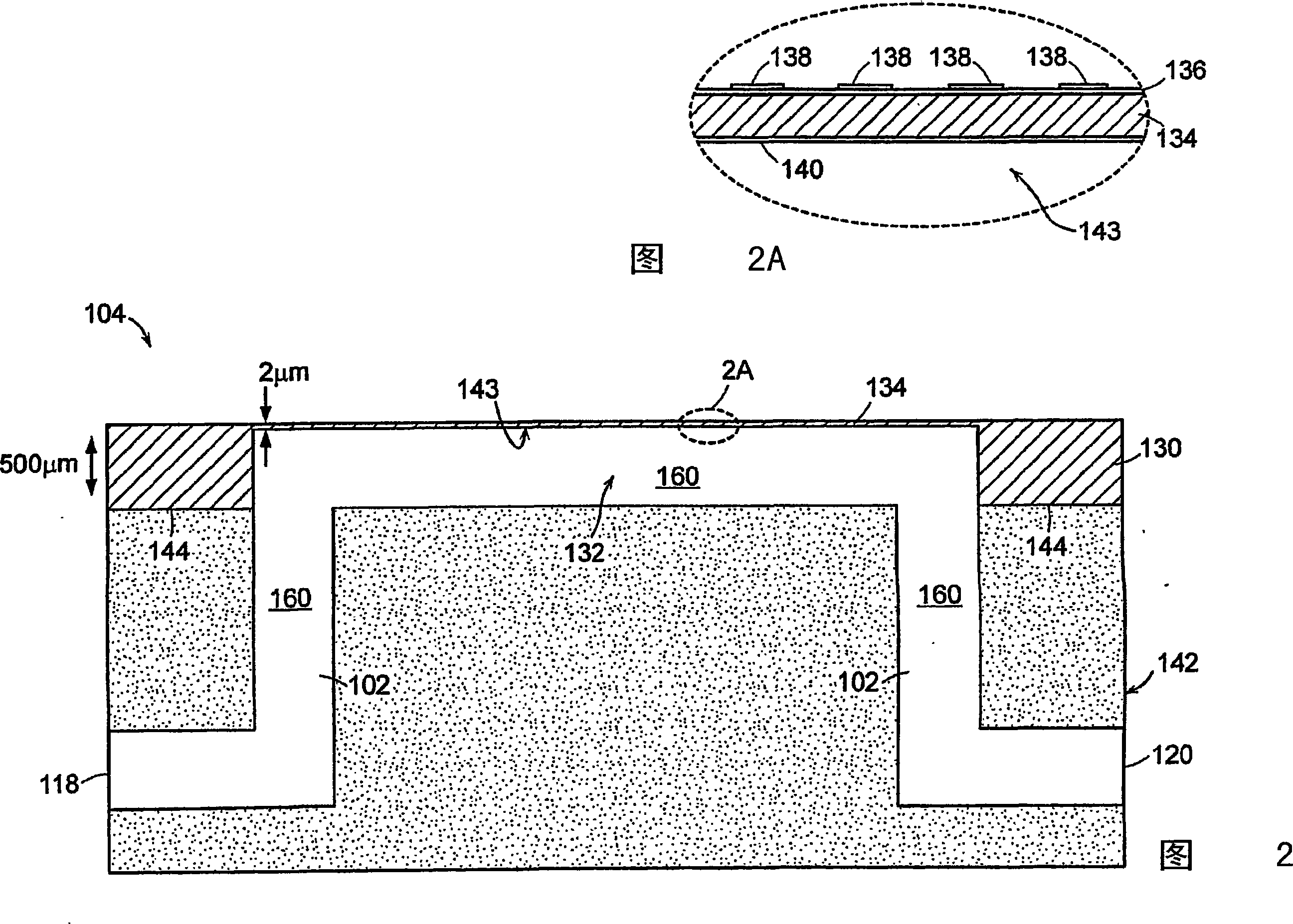
[0166]1. Deposit gold onto the surface of the flexible slab wave device 104 (eg sensor surface 143), clean the gold surface 143 with eg oxygen plasma.
[0167] 2. The ideal surface chemistry (characteristics) of the gold surface 143 should provide 1) reactive groups that prevent non-specific binding and 2) reside on the surface for covalent binding of capture agents. An exemplary surface chemistry (property) of the gold surface 143 is a self-assembled monolayer (SAM) of alkanethiols. SAMs can be formed from a mixture of two alkanethiols; one end containing a reactive group for subsequent covalent attachment to a capture agent, and one end containing a non-reactive group. For example, a C-terminated 11 EG of -alkanethiol 6 -OCH 2 COOH (EG6) and EG 3 Mixture of -OH(EG3). In one embodiment, the flexible plate wave device 104 (specifically, the surface 143 of the d...
Embodiment II
[0169] Example II: Using, for example, the method shown in Figure 3 to detect Escherichia coli O157:H7 in ground beef (Figure 5 contains data representing various concentrations of Escherichia coli)
[0170] 1. Prepare a sample containing E. coli O157:H7 analyte at a concentration greater than about 100 cfu / ml.
[0171] 2. Perform immunomagnetic separation to concentrate the analyte sample solution. This immunomagnetic separation can be performed using various commercially available instruments (eg, Pathatrix from Matrix Microsciences and Bead Retriever from Dynal Biotech) or by manual methods. Exemplary manual methods include:
[0172] a. Resuspend the magnetic beads coated with E. coli antibody (for example, Dynabeads anti-E. coli O157 purchased from Dynal Biotechnology) until the magnetic beads precipitated at the bottom of the test tube are dispersed. Place microcentrifuge tubes on a magnetic plate rack (eg, Dynal MPC-S). Pipette 1-20 μL of magnetic bead stock into this...
Embodiment III
[0191] Embodiment III: adopt, for example method steps shown in Figure 3 detect the prostate-specific antigen (PSA) in human serum
[0192] 1. Preparation of human serum analyte-containing samples obtained by centrifugation of human blood samples.
[0193] 2. Perform immunomagnetic separation to concentrate the analyte sample solution. This immunomagnetic separation can be performed using a variety of commercially available instruments (eg, Pathatrix from Model Microsciences and Bead Retriever from Dynal Biotechnology) or by manual methods. Exemplary manual methods include:
[0194] a. Resuspend the magnetic beads coated with PSA antibody (for example, Dynabeads anti-PSA purchased from Dynamic Biotechnology Company) until the magnetic beads precipitated at the bottom of the test tube are dispersed. Place microcentrifuge tubes on a magnetic plate rack (eg, Dynal MPC-S). Pipette 1-20 μL of magnetic bead stock into this tube (choose magnetic bead stock volume based on desired ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com