External diagnostic reagent kit used for measuring blood plasma fibrinogen FIB content
A technology for fibrinogen and in vitro diagnosis, which is applied in the direction of biological testing, microbial measurement/inspection, material inspection products, etc. It can solve the problems of loss of reliability of test results, reduce medical costs, save time, and have good consistency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Application of kit of the present invention
[0025] 1. Detection principle:
[0026] Adding thrombin to the plasma to be tested converts fibrinogen into insoluble fibrin, resulting in coagulation. When there is a sufficient amount of thrombin, it interacts with different contents of fibrinogen, and the coagulation time is negatively correlated with the content of fibrinogen, so the content of fibrinogen can be calculated.
[0027] 2. Detection steps (Pacific TS400C hemagglutination analyzer):
[0028] Dissolve FIB thrombin with a certain volume of distilled water by shaking gently. The FIB-rated plasma was reconstituted with 1.0ml of distilled water, and then diluted with buffer solution at 1:5, 1:10, 1:15, 1:20, and 1:30. Select the FIB item in the main menu of the coagulation analyzer for detection: take 100 μl of fixed-value plasma at different dilutions, pre-warm the pre-warmed well at 37°C for 3 minutes, transfer it into the measurement well and ac...
Embodiment 2
[0031] Embodiment 2 compares with commercially available FIB reagent stability
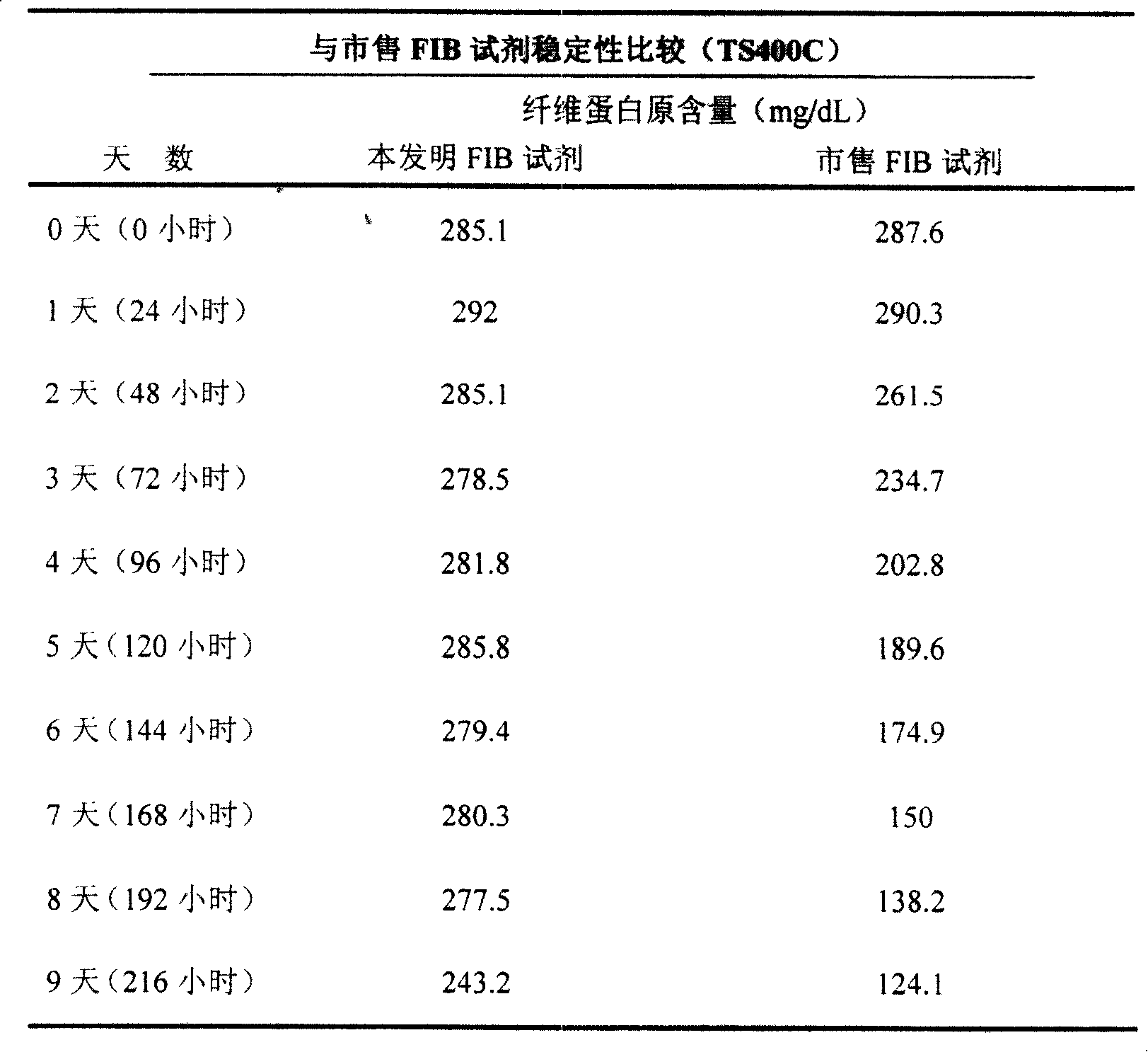
[0032] FIB thrombin was dissolved by shaking with a certain volume of distilled water, and then the reconstituted reagent was stored at 37°C. The same normal quality control plasma (FIB value: 200-400 mg / dL) was measured on a Pacific TS400C coagulation analyzer, and commercially available reagents were measured simultaneously. (See Table 2 for the results)
[0033] Table 2 shows that the FIB reagent of the present invention is relatively stable in the eight days after reconstitution, and it begins to change on the ninth day, while the commercially available reagents begin to change on the second day. This result is very important for clinical laboratories. Very important as it reduces waste and thus costs.
[0034] Table 2
[0035]
Embodiment 3
[0036] Embodiment 3 is compared with commercially available FIB reagent repeatability
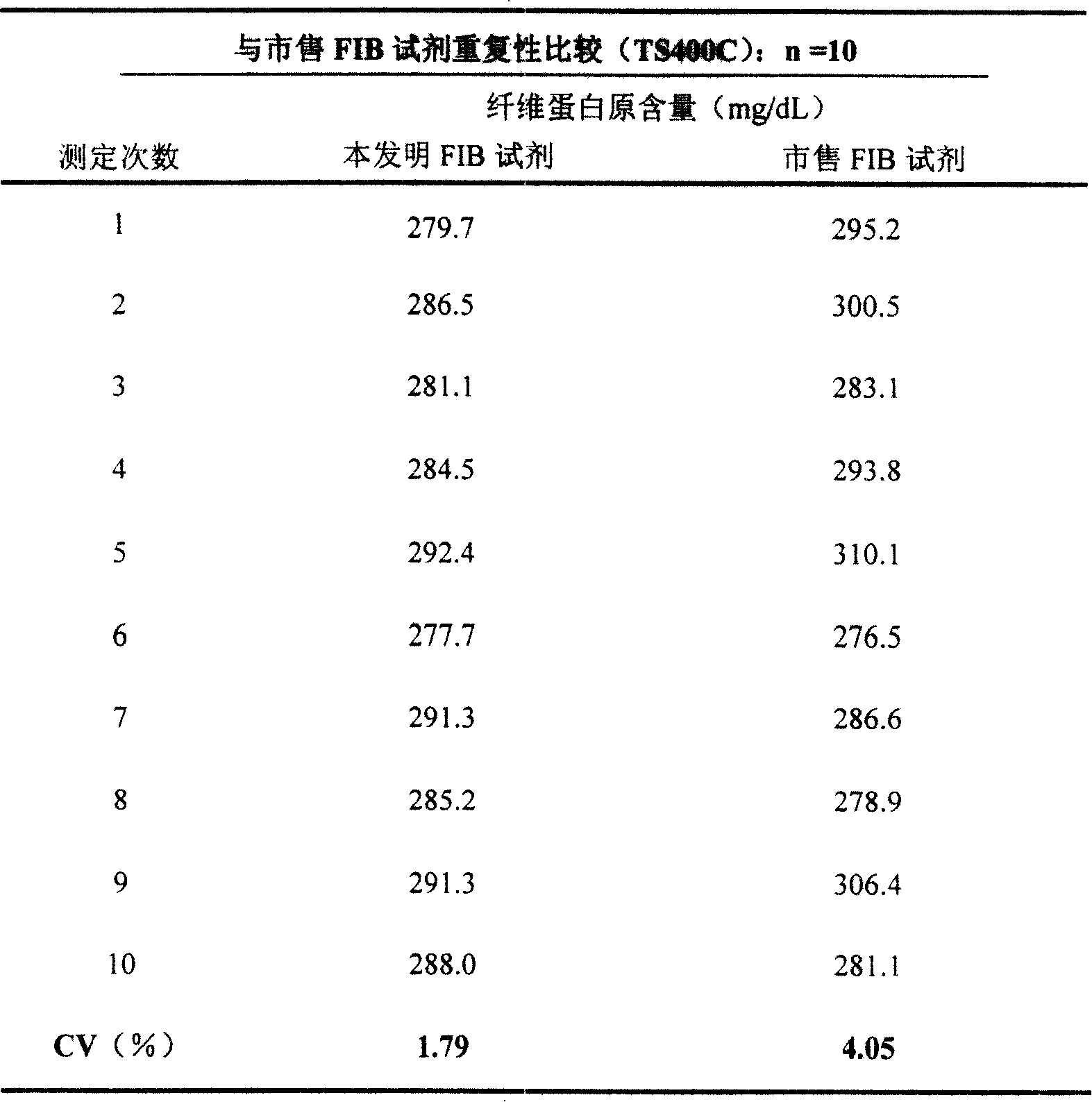
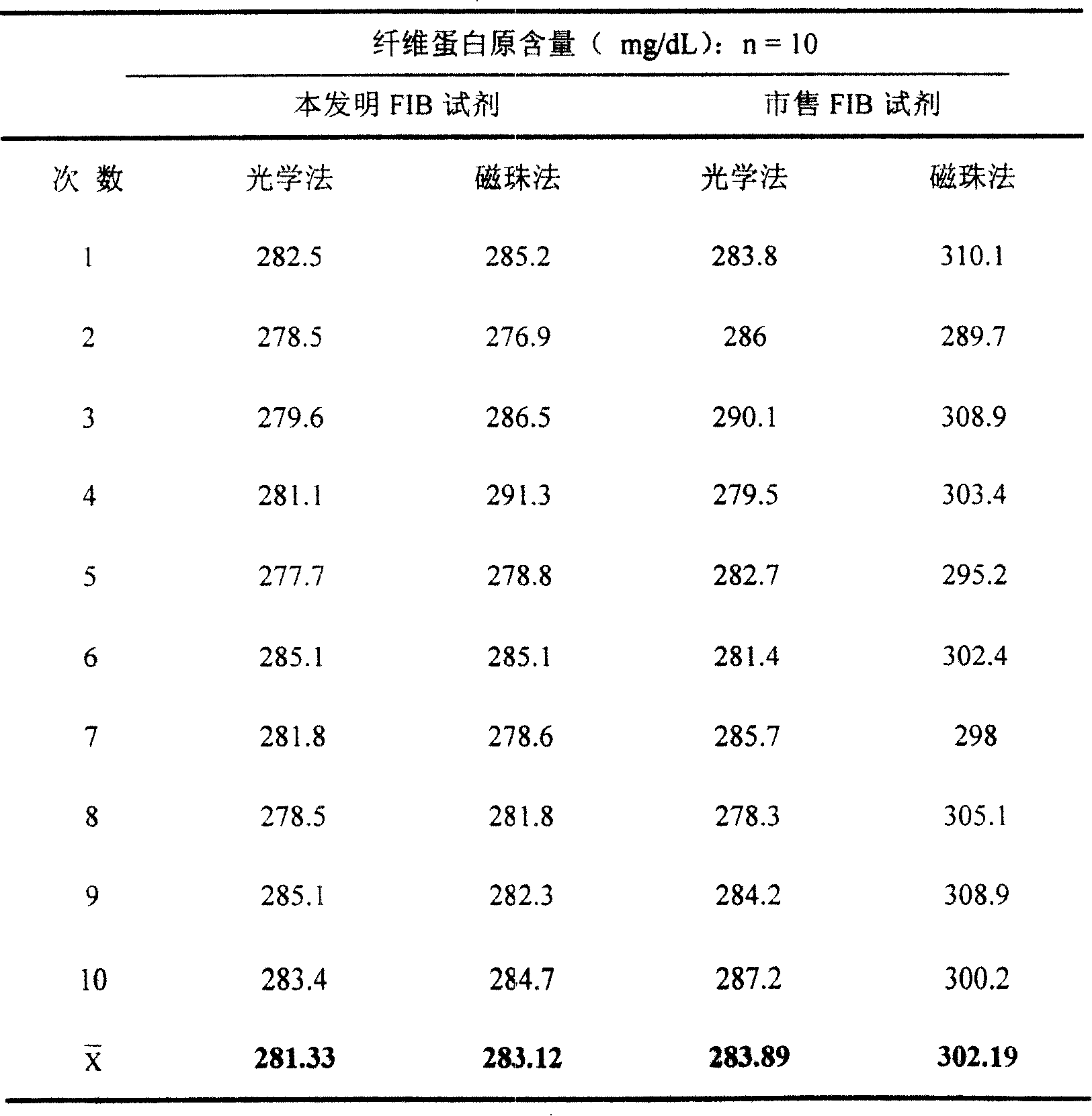
[0037] The FIB assay reagent of the present invention and the commercially available reagent were used to simultaneously measure the same sample on the Pacific TS400C coagulation analyzer according to the method described in Example 1. (See Table 3 for the results)
[0038] Table 3 shows that compared with commercially available reagents, the FIB reagent of the present invention has a smaller coefficient of variation (CV) and better repeatability.
[0039] table 3
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com