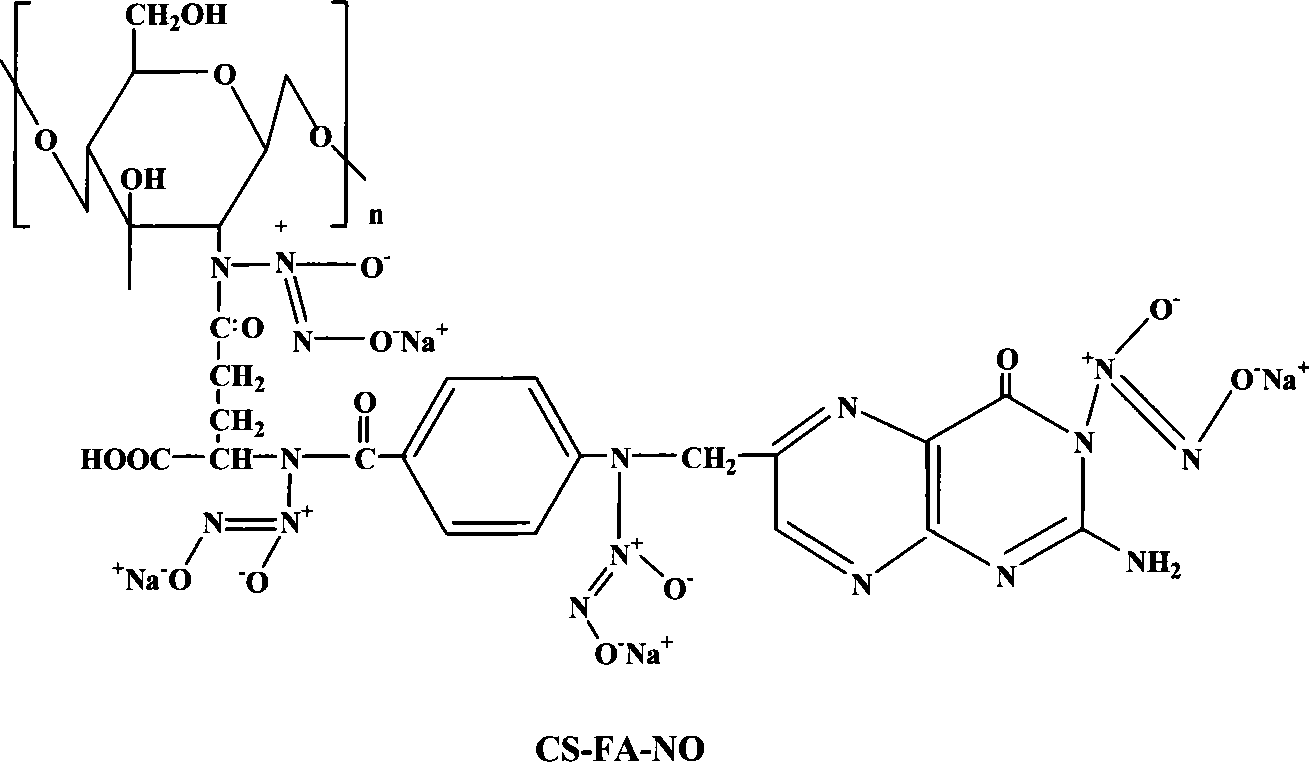
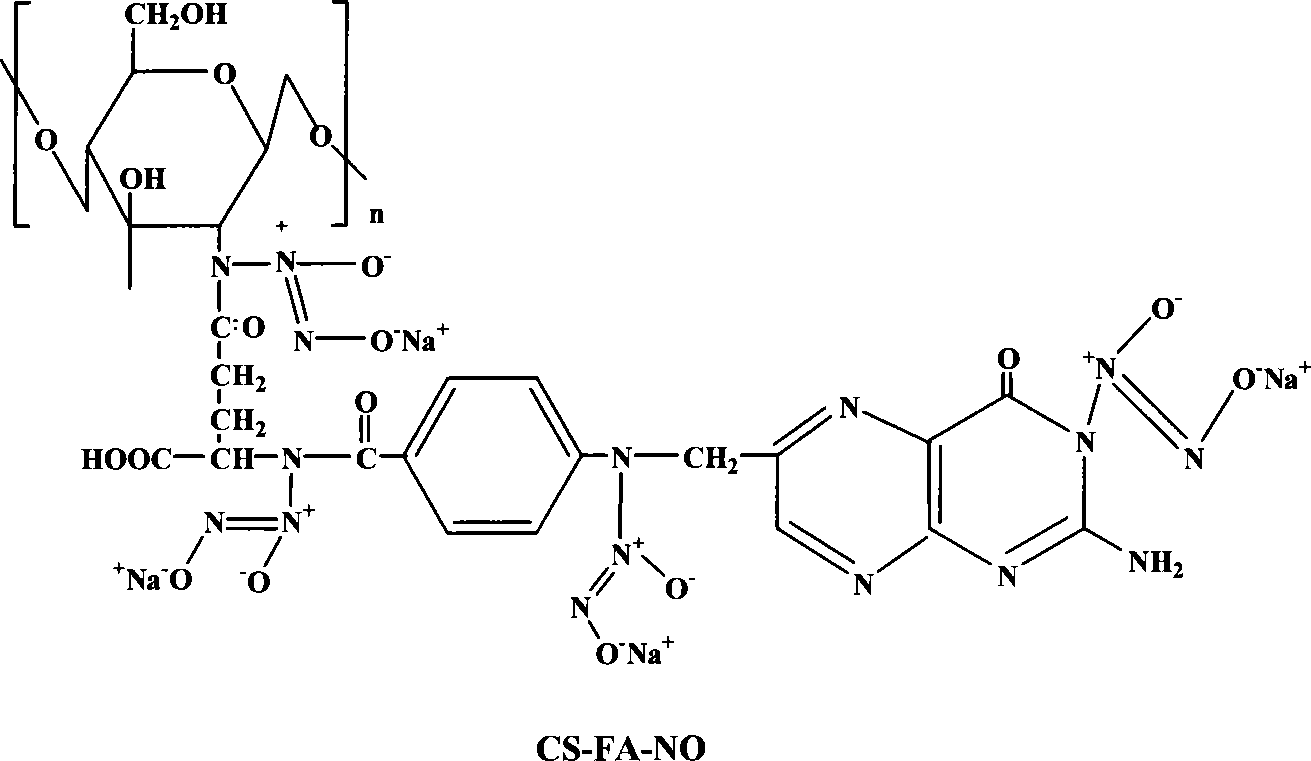
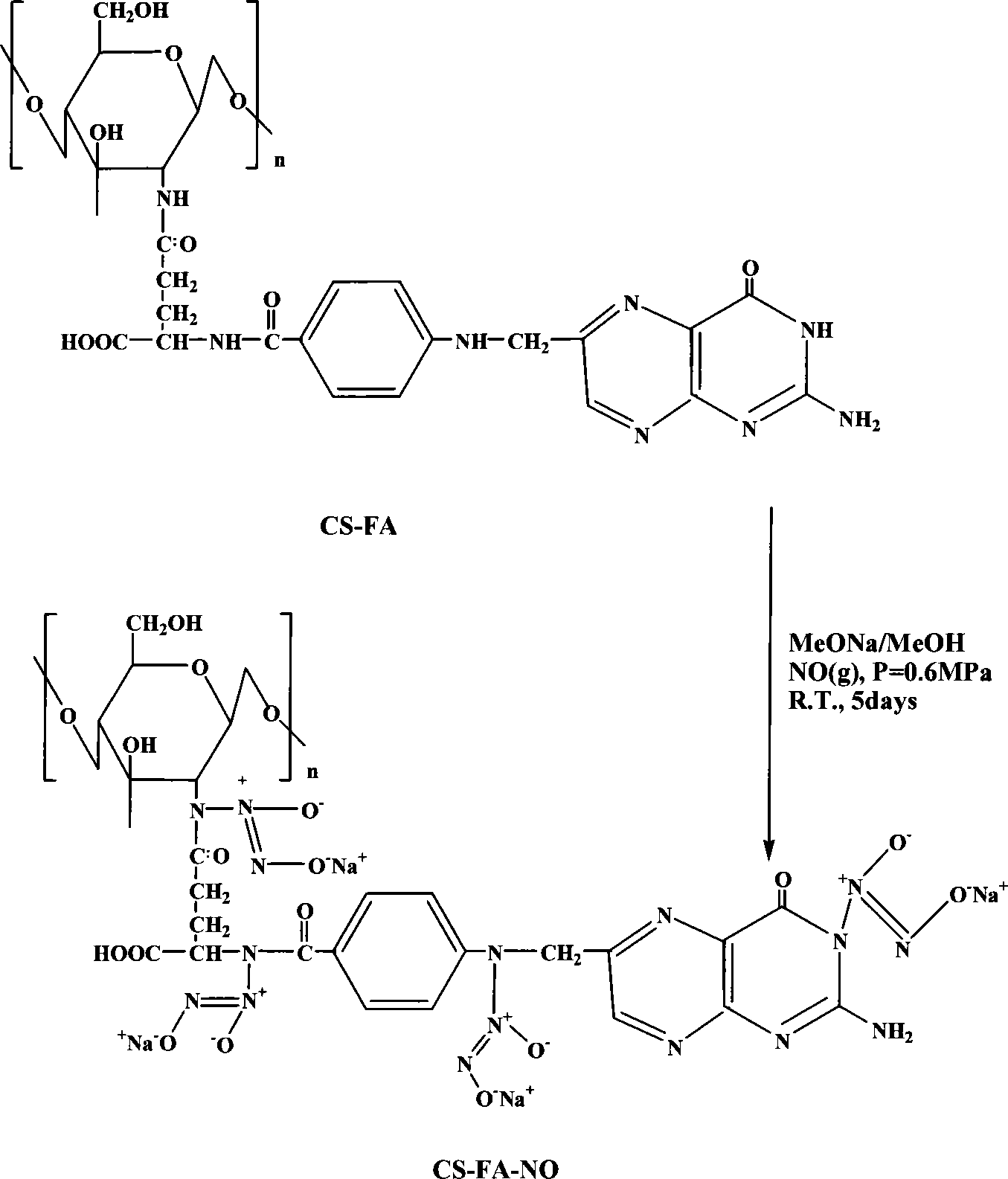
Folic acid modification of chitosan nucleophilic NO donator and compounding method thereof
A chitosan and modified technology, applied in the field of medical engineering, can solve problems such as low utilization rate, and achieve the effects of preventing restenosis, long release half-life, and promoting wound healing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the synthesis of folic acid (FA) modified 400,000 molecular weight chitosan (CS) / NO (molar ratio: FA / CS=2 / 1)
[0021] Accurately weigh 1.61g of chitosan (molecular weight: 400,000) sample, add 100ml of 1% acetic acid and stir until completely dissolved, adjust the pH value of the solution to 4.7, filter the solution with a disposable syringe filter and add it to a round bottom flask . Weigh 2.17g of folic acid and dissolve it in 30ml of dimethyl sulfoxide, stir it with magnetic force until it is completely dissolved, then add 0.5g of dicyclohexylcarbodiimide to the folic acid solution, and mix until it dissolves. The folic acid solution added with dicyclohexylcarbodiimide was added to the chitosan solution, and stirred and reacted in a water bath at 37° C. for 7 days. Adjust the pH value to 9.0 to terminate the reaction, dialyze the reaction product with a phosphate buffer solution with a pH value of 7.4 for 3 days, and then dialyze it with ultrapure wate...
Embodiment 2
[0024] Embodiment 2: the synthesis (molar ratio: FA / CS=1 / 1) of folic acid (FA) modified 1.24 million molecular weight chitosan (CS) / NO
[0025] Accurately weigh 1.61g of chitosan (molecular weight: 1.24 million) sample, add 100ml of 1% acetic acid and stir until completely dissolved, adjust the pH value of the solution to 4.7, filter the solution with a disposable syringe filter and add it to a round bottom flask . Weigh 4.34g of folic acid and dissolve it in 30ml of dimethyl sulfoxide, stir it with magnetic force until it is completely dissolved, then add 0.5g of dicyclohexylcarbodiimide to the folic acid solution, and mix until it dissolves. The folic acid solution added with dicyclohexylcarbodiimide was added to the chitosan solution, and stirred and reacted in a water bath at 37° C. for 7 days. Adjust the pH value to 9.0 to terminate the reaction, dialyze the reaction product with a phosphate buffer solution with a pH value of 7.4 for 3 days, and then dialyze it with ultr...
Embodiment 3
[0028] Embodiment 3: the synthetic (molar ratio: FA / CS=1 / 2) of folic acid (FA) modified 1.88 million molecular weight chitosan (CS) / NO
[0029] Accurately weigh 1.61g of chitosan (molecular weight 1.88 million) sample, add 100ml of acetic acid and stir until completely dissolved, adjust the pH value of the solution to 4.7, filter the solution with a disposable syringe filter and add it to a round bottom flask. Weigh 8.68g of folic acid and dissolve it in 30ml of dimethylsulfoxide, stir it with magnetic force until it is completely dissolved, then add 0.5g of dicyclohexylcarbodiimide to the folic acid solution, and mix until it dissolves. The folic acid solution added with dicyclohexylcarbodiimide was added to the chitosan solution, and stirred and reacted in a water bath at 37° C. for 7 days. Adjust the pH value to 9.0 to terminate the reaction, dialyze the reaction product with a phosphate buffer solution with a pH value of 7.4 for 3 days, and then dialyze it with ultrapure w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com