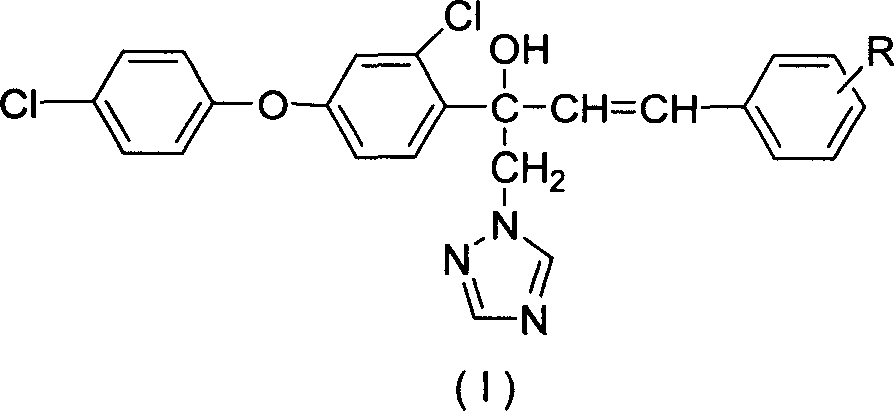
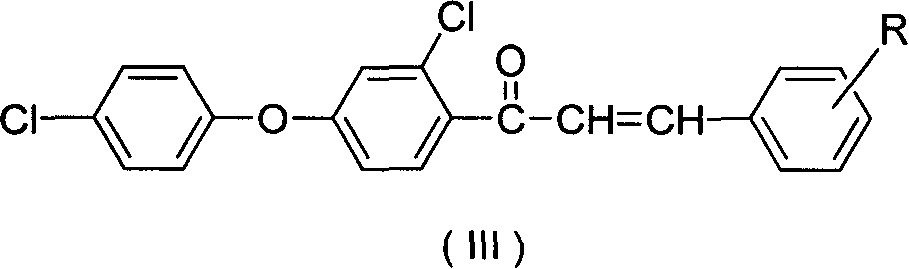
Synthesis of compound containing aryl ether triazole
A technology of bactericidal activity and compounds, applied in the directions of fungicides, biocides, organic chemistry, etc., can solve the problems of inhibition of strawberry growth, single action mechanism and action site, loss of high efficiency, etc., and achieve significant specific killing activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
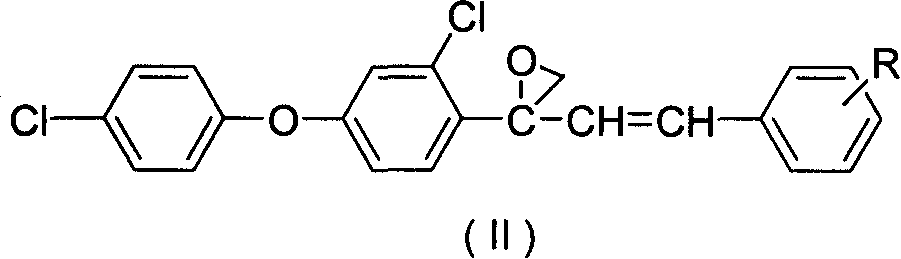
[0017] Example 1: Preparation of 2-(2-chloro-4-(4-chlorophenoxy)phenyl)-2-styryl propylene oxide
[0018] Take 3.68g (0.01mol) of 1-(2-chloro-4-(4-chlorophenoxy)phenyl)-3-phenylprop-2-en-1-alcohol, 2.26g ( 0.012mol) and 40 mL of diethyl ether were put into a 100 mL three-necked flask, and 2.69 g of potassium hydroxide powder was added under cooling and stirring in an ice bath, and then refluxed and stirred for 6 hours, and the reaction solution was neutralized to PH=7 with 30% dilute sulfuric acid, diethyl ether Extracted, dried and evaporated to obtain 3.59 g of light yellow solid 2-(2-chloro-4-(4-chlorophenoxy)phenyl)-2-styryl propylene oxide, yield 94.1%, melting point: 79 ~82°C.
Embodiment 2
[0019] Example 2: 2-(2-chloro-4-(4-chlorophenoxy)phenyl)-4-phenyl-1-(1H-1,2,4-triazol-1-ylmethyl) - Preparation of but-3-en-2-ol
[0020] Add 3.82g (0.01mol) of 2-(2-chloro-4-(4-chlorophenoxy)phenyl)-2-styryl propylene oxide, 0.8g (0.012mol) of triazole into a 50mL single-necked flask ), catalyst A and N,N-dimethylformamide 20mL, reflux reaction for 6h, the reaction solution was poured into water, the solvent was extracted, subtracted and evaporated to obtain a yellow viscous liquid, and recrystallized with acetone to obtain a yellow solid 2-(2- Chloro-4-(4-chlorophenoxy)phenyl)-4-phenyl-1-(1H-1,2,4-triazol-1-ylmethyl)-but-3-ene-2- Alcohol, 3.52g, yield 78.0%, melting point 142-145°C.
[0021] Other target compounds (I) can be prepared by a method similar to the above. Listed in Table 1 are some compounds synthesized by the present invention.
[0022]
[0023]
[0024] The aryl ether triazole compounds synthesized by the present invention have very slight specific in...
Embodiment 3
[0025] Embodiment 3 antibacterial activity test
[0026] (1) Experimental method: using the isolated plate method
[0027] 1. Materials and methods
[0028] (1) Test strain
[0029] In this experiment, the following common agricultural plant fungal diseases were selected: wheat head blight (G.Zease), tomato early blight (A.Solani), asparagus stem blight (R.Solani), apple ring spot (P.Pircola), peanut Brown spot (C. Arachidicata).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com