Method for preparing high-purity magnesium hydroxide by using atomized ammonia as precipitator
A technology of magnesium hydroxide and precipitating agent, applied in magnesium hydroxide and other directions, can solve the problems of high free ammonia concentration and environmental pollution, and achieve the effects of high purity, less investment and more specifications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
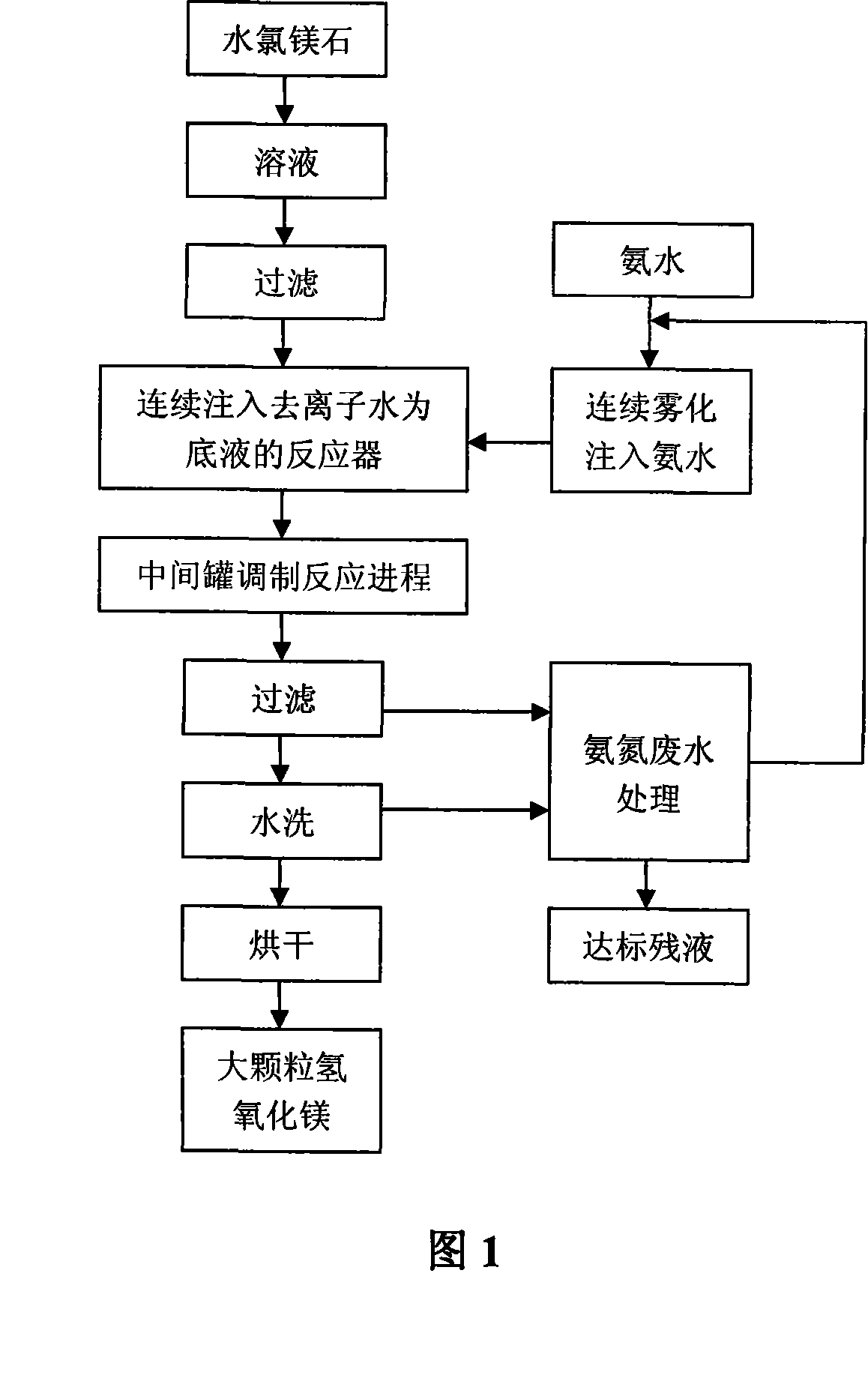
Image
Examples
Embodiment 1
[0036] Continuous
[0037] (1) preparation concentration is the magnesium chloride solution of 2mol / L:
[0038] Add bischofite to deionized water, stir at room temperature until completely dissolved, and prepare a magnesium chloride solution with a concentration of 2 mol / L; use a filter press to filter the solution to remove impurities;
[0039] (2) Inject the reaction bottom solution
[0040] Inject 15 liters of distilled water into a reactor with an effective volume of 30 liters as the bottom liquid, add 100 g of seed crystals, heat to 85 ° C and keep it warm;
[0041] (3) Inject atomized ammonia water
[0042] Continuously inject atomized ammonia water with a mass concentration of 10% at a ratio of 1:2 to magnesium ions.
[0043] (4) inject magnesium chloride solution
[0044] 10 minutes after the injection of atomized ammonia, the magnesium chloride solution with a concentration of 3mol / L was continuously injected into the reactor at a flow rate of 40 liters per hour, ...
Embodiment 2
[0049] Intermittent
[0050] (1) preparation concentration is the magnesium chloride solution of 4.5mol / L:
[0051] Add bischofite to deionized water, stir at 50°C until completely dissolved, and prepare 15 liters of magnesium chloride solution with a concentration of 4.5mol / L; use a filter press to filter the solution to remove impurities;
[0052] (2) Inject the reaction bottom solution
[0053] Inject 15 liters of distilled water into a reactor with an effective volume of 30 liters as the bottom liquid, add 100 g of seed crystals, heat to 85°C and keep it warm;
[0054] (3) Inject atomized ammonia water
[0055] Continuously inject atomized ammonia water with a mass concentration of 25% at a ratio of 1:2 to magnesium ions.
[0056] (4) inject magnesium chloride solution
[0057] 5 minutes after atomized ammonia began to inject, the magnesium chloride solution with a concentration of 4.5mol / L was continuously injected into the reactor at a flow rate of 30 liters per hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com