Timely released preparation and preparation thereof
A technology of timed release and preparation, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc. It can solve the problems of difficulty in making soft materials, short time lag, etc., achieve good pulse effect, reduce viscosity, and prevent rapid release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
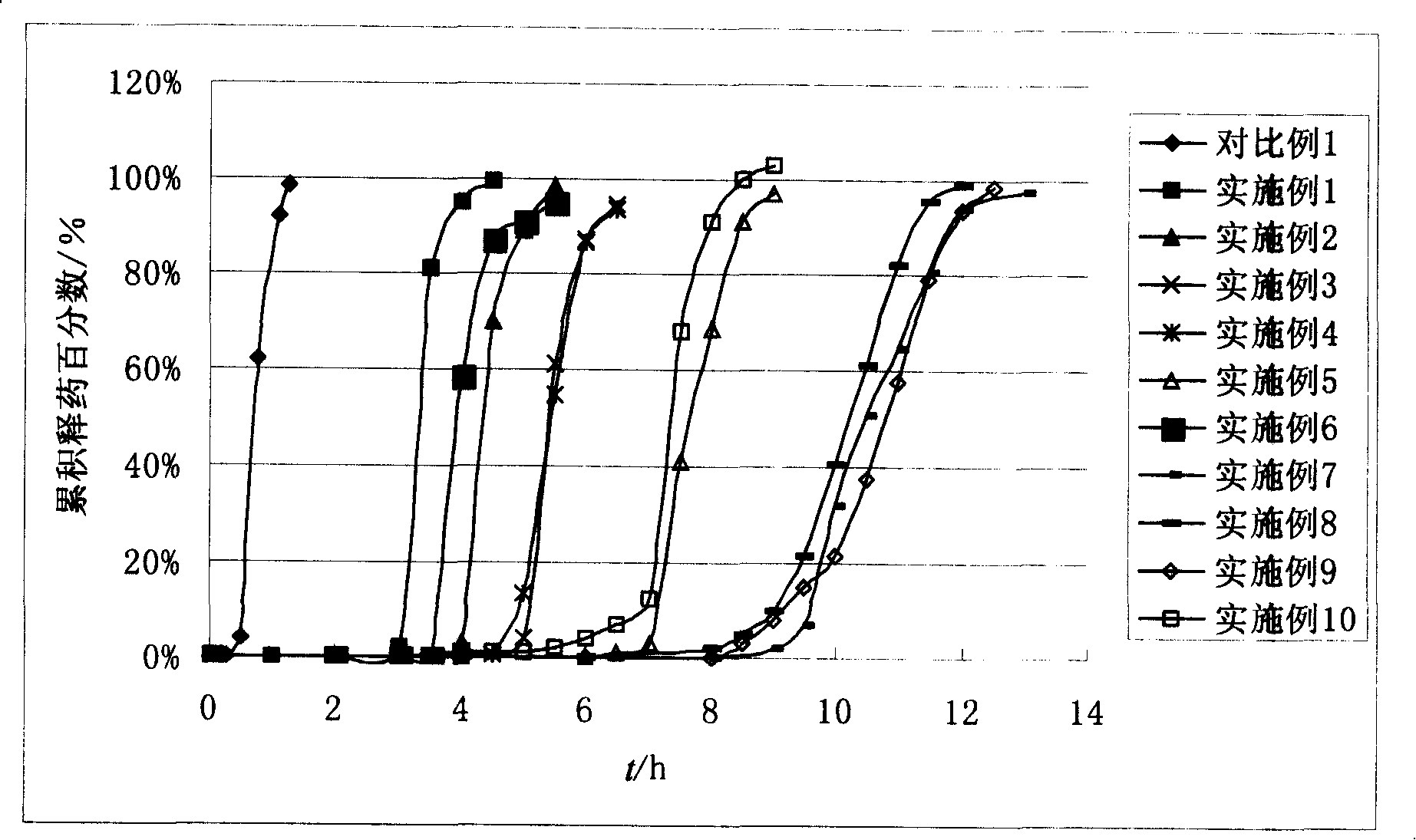
Examples
Embodiment 1
[0021] Tablet core prescription (total amount is 100mg):
[0022] %(W / W)
[0023] Isosorbide mononitrate 20
[0024] Microcrystalline Cellulose 20
[0025] Starch 46.2
[0026] Crospovidone 10
[0027] Povidone 2.8
[0029]
[0030] Total 100
[0031] After mixing the above-mentioned first 4 ingredients, add 28 μl of 75% ethanol aqueous solution containing 10wt% povidone to make a soft material, sieve it, put it into an oven to dry at 50°C for 1 hour, add 1wt% talcum powder and mix for 3 minutes, Compressed into tablet cores.
[0032] Coating prescription (300mg in total):
[0033] %(W / W)
[0034] Hydroxypropyl Methyl Cellulose K100M 10
[0035] Acrylic resin RL 31.5
[0036] Povidone K30 18
[0037] Microcrystalline Cellulose 15
[0038] Lactose 22.5
[0040] Talc powder 1
[0041] ...
Embodiment 2
[0045] Tablet core prescription (total amount is 100mg):
[0046] %(W / W)
[0047] Nitrindipine 10
[0048] Lactose 39.2
[0049] Mannitol 15
[0050] Sodium carboxymethyl starch 8
[0051] Microcrystalline Cellulose 25
[0052] Hydroxypropyl methylcellulose 1.8
[0053] Talc powder 1
[0054]
[0055] Total 100
[0056] After mixing the aforementioned 5 ingredients, add 60 μl of 50% ethanol aqueous solution containing 3% hydroxypropyl methylcellulose to make a soft material, sieve it, dry it at 50°C for 1 hour, add 1 wt% talcum powder and mix for 3 minutes , pressed into tablet cores.
[0057] Coating prescription (300mg in total): % (W / W)
[0058] Hydroxypropyl Methyl Cellulose K15M 10
[0059] Povidone K30 18
[0060] Ethyl cellulose (0.01Pa.s) 30
[0061] Microcrystalline Cellulose 15
[0062] starch 23
[0064] Talc powder 1
[0065] ...
Embodiment 3
[0069] Tablet core prescription (total amount is 100mg):
[0070] %(W / W)
[0071] Salbutamol Sulfate 8
[0072] Starch 73.4
[0073] Sodium Carboxymethyl Cellulose 15
[0074] Povidone 2.6
[0075] Talc powder 1
[0076]
[0077] Total 100
[0078] After mixing the above three ingredients, add 26 μl of 75% ethanol aqueous solution containing 10% povidone to make a soft material, sieve, dry at 50°C for 1 hour, add 1wt% talcum powder and mix for 3 minutes, and press into Chips.
[0079] Coating prescription (300mg in total): % (W / W)
[0080] Hydroxyethylcellulose (Natrosol HX) 6
[0081] Povidone K30 25
[0082] Ethyl cellulose (0.01Pa.s) 30
[0083] Microcrystalline Cellulose 12
[0084] Compressible starch 22
[0085] Zinc stearate 4
[0086] Talc powder 1
[0087]
[0088] Total 100
[0089] After uniformly mixing the first...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com