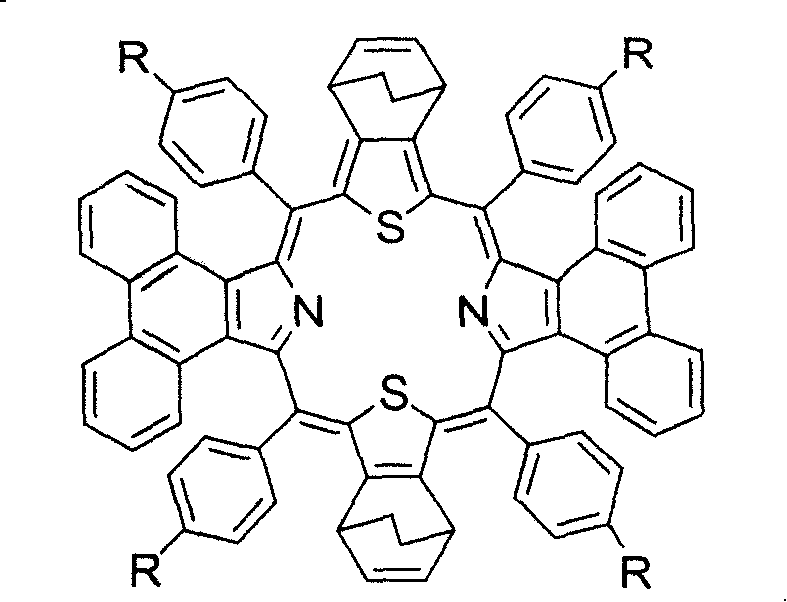
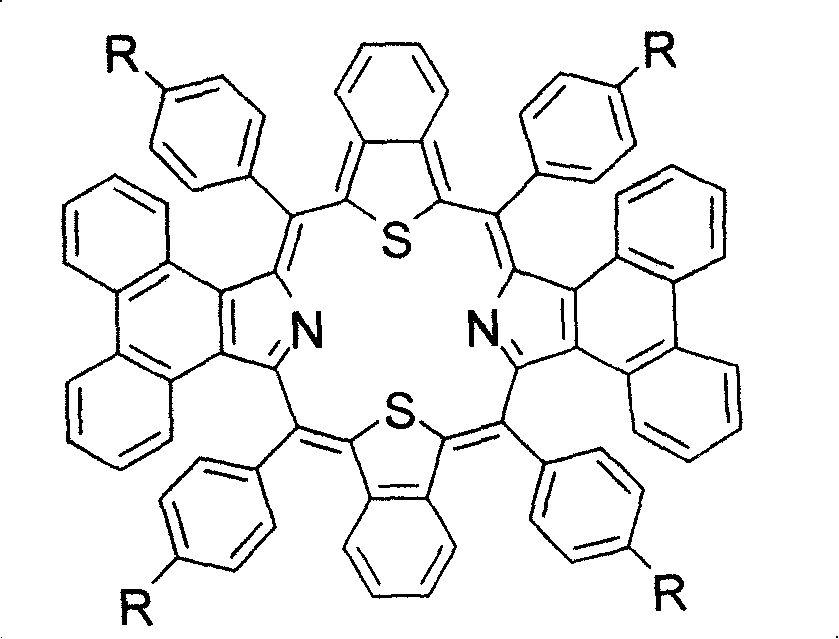
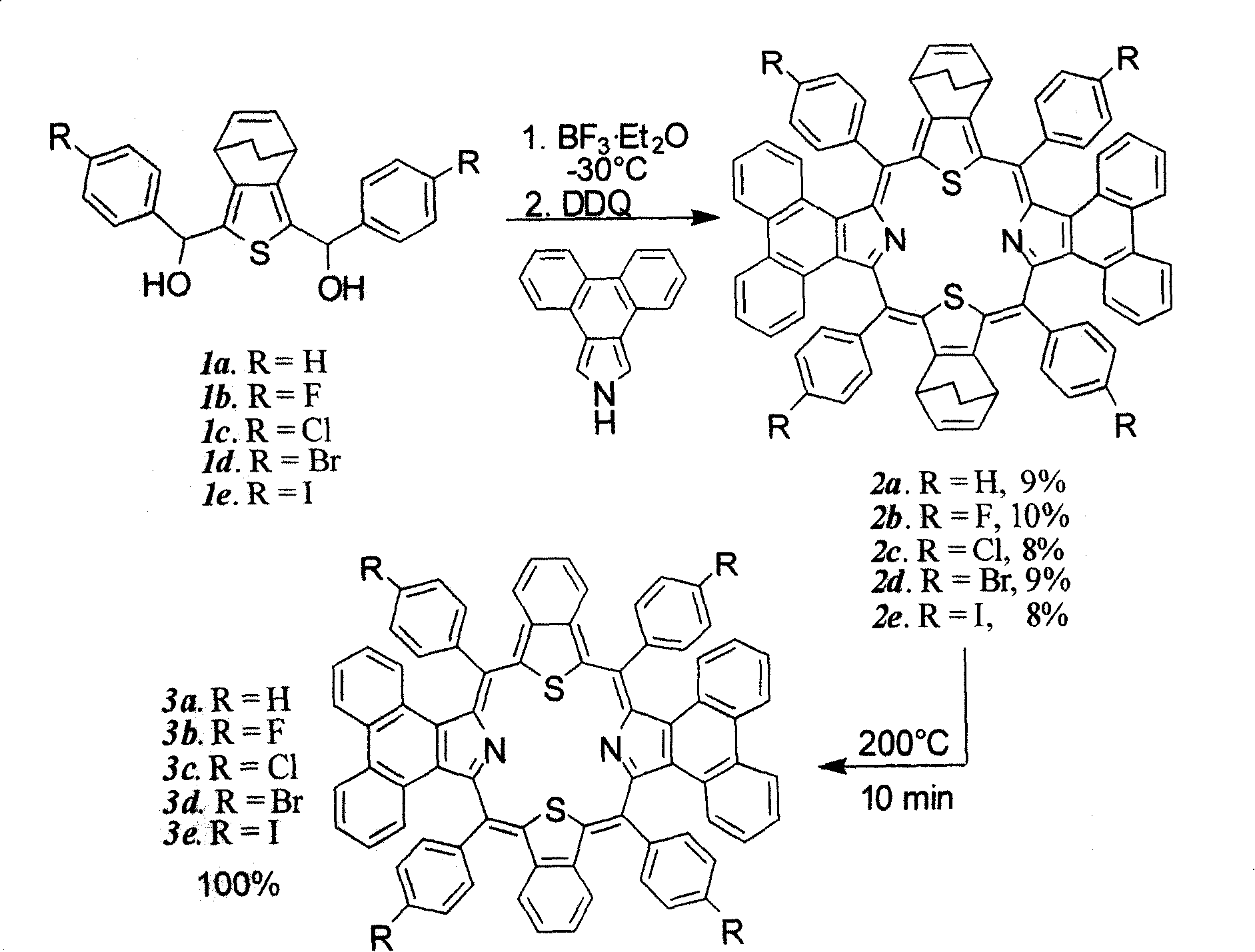
Preparation and application of center modified porphyrin derivatives with two different kinds of aromatic ring conjugation
A derivative, a technology of dithioporphyrin, is applied in the preparation and application of a class of center-modified porphyrin derivatives with two different aromatic ring conjugates, to achieve the effect of improving absorption efficiency and conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Synthesis of meso-circular (4-fluorophenyl)-diphenanthrene-two bicyclo-dithioporphyrin derivatives (2b):
[0036] In a 250ml round bottom flask, add 1mmol (410mg) 2,5-bis((4-fluorophenyl)hydroxymethyl)-bicyclothiophene (1b), 1mmol (217mg) phenanthropyrrole and 100ml anhydrous dichloromethane , put in a magnet and start stirring, put the reaction bottle into a cryogenic device under the protection of argon and avoid light, control the reaction temperature at -30°C, add a total of 80 μl of BF 3 ·Et 2 O, make it react at low temperature for 1 hour, then allow it to naturally heat up to 25° C., and continue the reaction for 48 hours. 1 mmol (227 mg) of DDQ was added to the reaction solution and reacted for 2 hours. The solvent was distilled off under reduced pressure for chromatographic separation, and the purple-black product 2b was obtained after recrystallization from methanol and chloroform. Yield: 10%; melting point > 120°C (decomposition); UV-vis (CHCl ...
Embodiment 2
[0037] Example 2: 5,10,19,28-Tetrakis(4-fluorophenyl)-diphenanthrene[9,10-b:9,10-l]-dibenzo[g:q]-30,32 - Synthesis of dithioporphyrin (3b):
[0038] In a 25ml round-bottomed flask filled with 0.1mmol (118mg) of compound 2b, vacuumize with an oil pump and heat to 220°C. After 30 minutes, compound 2b is completely converted into compound 3b, with a yield of 100%, and a melting point > 300°C; UV -vis(CHCl 3 ): λ max (ε×10 4 )=543(2.1), 645(0.23), 712(0.53), 869(0.15)nm( Figure 4) ; 1 H NMR (500MHz, CDCl 3 , 25°C, TMS) δ7.039(m, 8H), 7.295(m, 8H), 7.591(m, 8H), 7.759(m, 4H), 8.113(m, 4H), 8.539(m, 4H), 8.996(m, 4H); HRMS m / z experimental value: 1121.2660, [M+H] + , C 76 h 41 f 4 N 2 S 2 Theoretical value: 1121.2642 ( Figure 6) .
Embodiment 3
[0039] Example 3: Synthesis of meso-tetraphenyl-diphenanthrene-two bicyclo-dithioporphyrin derivatives (2a):
[0040] The preparation method is the same as in Example 1, except that 1 mmol (374 mg) of 2,5-diphenylhydroxymethyl-bicyclothiophene (1a) is added. Yield: 9%; Melting point > 200°C (decomposition); UV-vis (CHCl 3 ): λ max (ε×10 4 )=534(3.04), 608(0.56), 672(0.33), 862(0.22)nm; HRMSm / z experimental value: 1105.3690, [M+H] + , C 80 h 53 N 2 S 2 Theoretical value: 1105.3668.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com