CEA negative colorectal cancer detection and prognosis judgement mass spectrum reagent kit and method
A colorectal cancer and kit technology, applied in the field of protein detection, can solve the problems of difficult to obtain reproducibility of pathological evidence, lack of early monitoring methods, and no obvious clinical manifestations of tumors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Identification of normal and CEA-negative colorectal cancer patients and preparation of mass spectrometry kits
[0064] (1) Experimental method
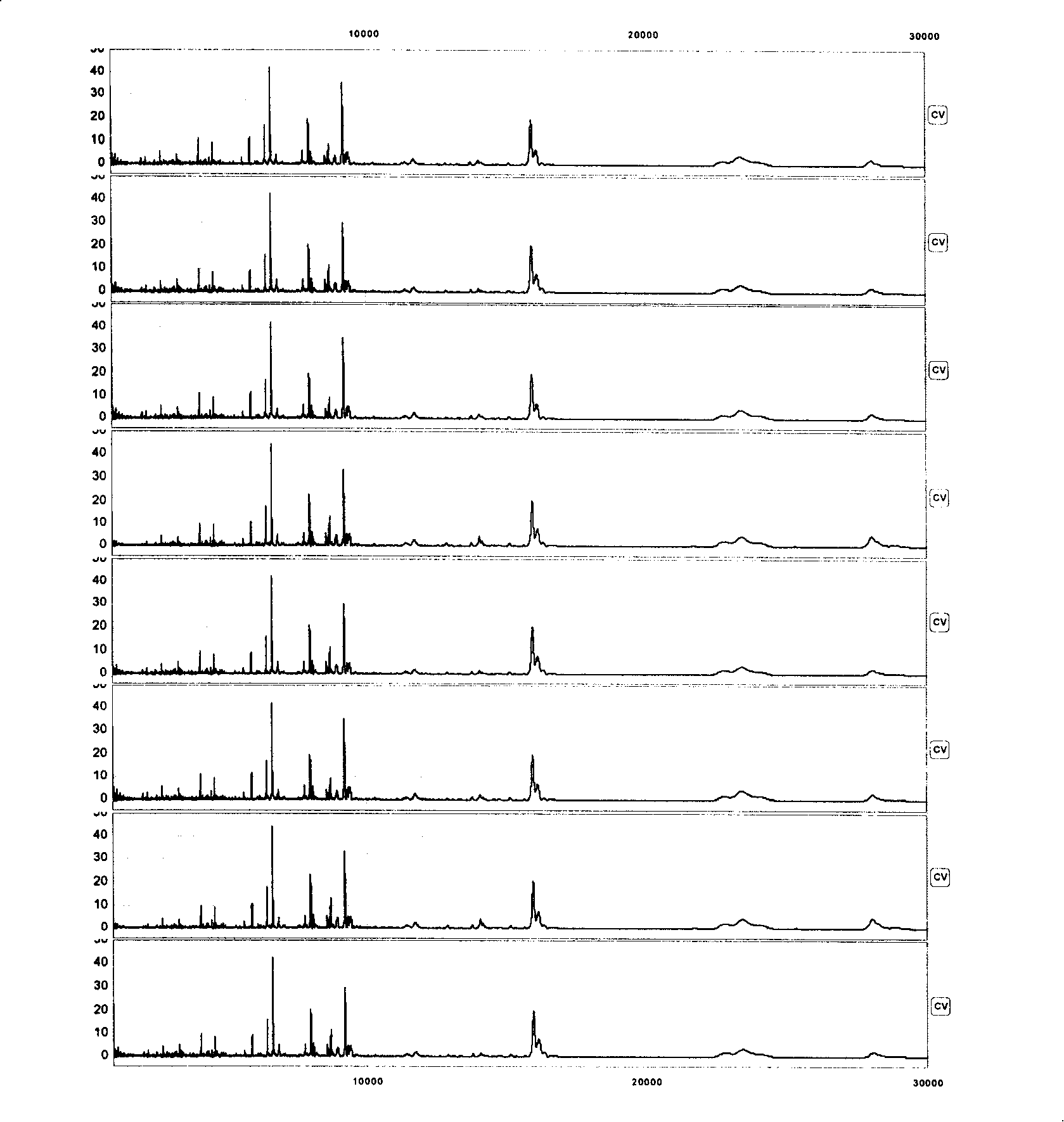
[0065]60 patients with CEA-negative colorectal cancer (CEA≤5μg / L) and 68 patients with CEA-positive colorectal cancer (CEA>5μg / L), all of them were adenocarcinoma, with an average age of 61.5 years; only 20 patients with advanced colorectal cancer No lymph node metastasis was seen in 1 case, and the rest were associated with lymph node metastasis; 32 cases had organ metastasis: 16 cases of liver metastasis, 12 cases of lung metastasis, and 4 cases of ovarian metastasis. Fifteen patients with colorectal benign tubular adenoma confirmed by colonoscopy. 60 healthy subjects, with an average age of 59.5 years, were from a population with normal liver function and renal function. Collect 1mL of venous blood from the subject on an empty stomach, immediately after collection, let it stand in a refrigerator at 4°C for 2 hou...
Embodiment 2
[0082] The double-blind test of embodiment 2 kit
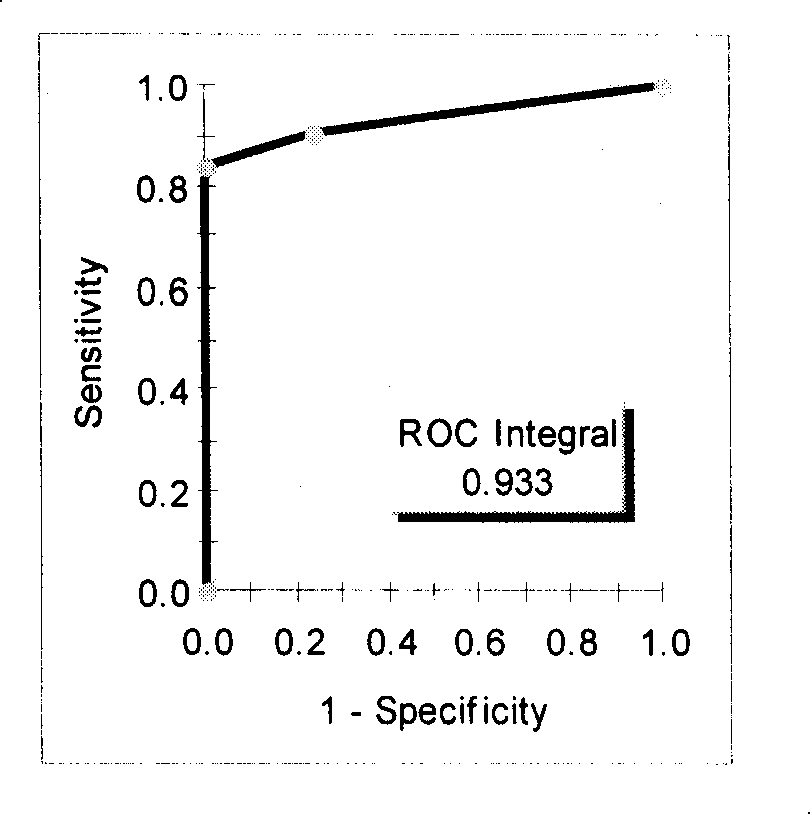
[0083] Several characteristic protein peaks were screened out from Example 1, and the test model composed of 3 differential peaks of 3398.3, 5477.1, and 8453.9 ± 15Da had a 100% sensitivity to the blind screening test of carcinoembryonic antigen-negative colorectal cancer patients and healthy people , specificity 83% (Table 2); that is, when 3398.3Da is less than or equal to 7.83; or when 3398.3Da is greater than 7.83 and 5477.1Da is greater than 1.54 and 8453.9Da is greater than 1.18, it is a colorectal cancer patient with negative expression of carcinoembryonic antigen, otherwise it is a healthy population ( image 3 , Table 1, 2).
[0084] Table 2 Double-blind test of kits
[0085] double blind test
Carcinoembryonic antigen-negative colorectal cancer (60)
normal(60)
carcinoembryonic antigen negative colorectal cancer
60
10
normal
0
50
[0086] Sensitivity 100...
Embodiment 3
[0088] Example 3 Screening of colorectal cancer metastasis-associated polypeptides
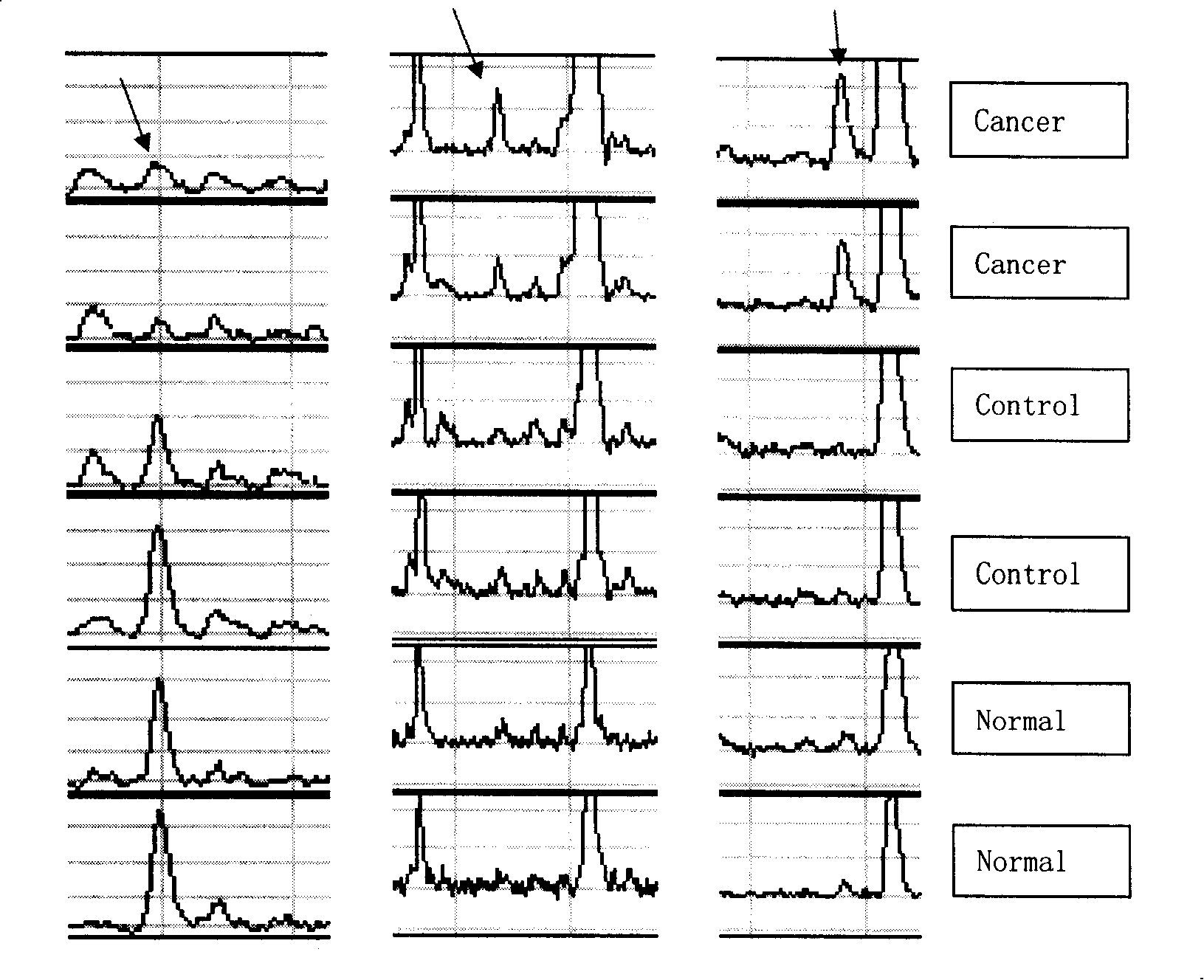
[0089] Patients with colorectal cancer were grouped according to organ metastasis, and then compared between groups, among which 4 polypeptides and proteins were significantly increased in the organ metastasis group (Table 3). The colorectal cancer patients were grouped according to the lymph node metastasis group, and it was found that the two polypeptides and proteins were significantly increased in the lymph node metastasis group (Table 4). The 11683.3Da peak of the same representative patient shows that the sensitivity of WCX magnetic beads is higher than that of WCX chips ( Figure 4 ).
[0090] Table 3 Differences in the abundance of polypeptide proteins between colorectal cancer organ metastasis group and non-organ metastasis group (P<0.05, x±sd)
[0091]
[0092] Table 4 Differences in polypeptide protein abundance between colorectal cancer lymph node metastasis group and non-lymp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com