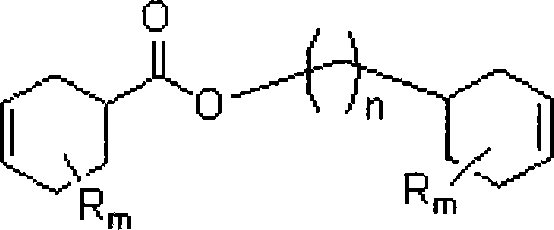
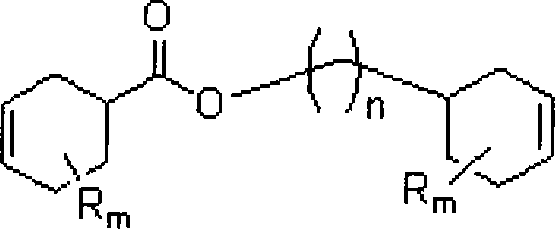Method for synthesizing aliphatic diepoxides
A technology of a diepoxide compound and a synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of difficult product separation, large system, low efficiency, etc., and achieve the effects of improving safety, improving efficiency, and inhibiting hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
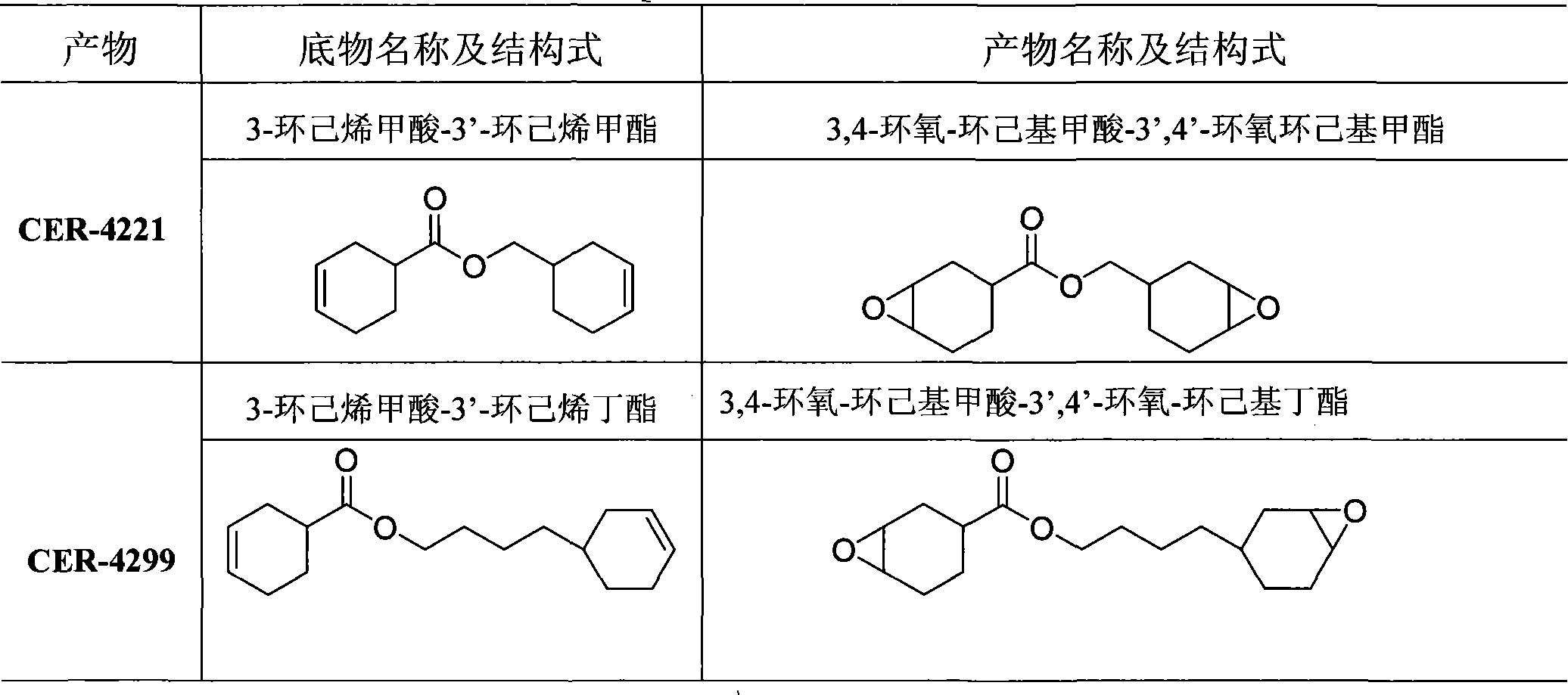
[0041] Catalytic double epoxidation synthesis of embodiment 1 CER-4221
[0042] 44g of 3-cyclohexenecarboxylic acid-3'-cyclohexene methyl ester, 2.6g of [C 16 h 33 N(CH 3 ) 3 ] 3 [W 2 o 3 (O 2 ) 4 ], 88g dichloroethane heated and stirred to 50 degrees together. Add 35% hydrogen peroxide (H 2 o 2 0.5mol, 2.5eq), the reaction is exothermic. The reaction was stirred for 1 hour, and a sample was taken for detection. The conversion rate is 99%, and the ratio of single to double is 1:18. After cooling to room temperature, the aqueous layer was separated. The organic layer was washed three times with saturated brine, dried over anhydrous sodium sulfate, passed through a short silica gel column, and concentrated to obtain 33.5 g of a colorless or pale yellow viscous liquid. The yield is 67%, and the epoxy equivalent is about 154.
example 2
[0043] Catalytic double epoxidation synthesis of example 2 CER-4221
[0044] 44g of 3-cyclohexenecarboxylic acid-3'-cyclohexene methyl ester, 3.3g of [C 5 h 5 NC 16 h 33 ] 2 [W 2 o 3 (O 2 ) 4 ], 88g chloroform is heated and stirred to 50 degrees together. 25% hydrogen peroxide (H 2 o 2 After the pH value of 0.5mol, 2.5eq) is adjusted to about 4, it is added dropwise in the reaction solution, and the reaction exotherm is violent. The reaction was stirred for 1 hour, and a sample was taken for detection. The conversion rate is 99%, and the ratio of single to double is 1:18. Cool to room temperature and separate the liquids. The organic layer was washed three times with water, dried over anhydrous sodium sulfate, passed through a short silica gel column, and concentrated to obtain 38.8 g of a colorless or pale yellow viscous liquid. The yield is 77%, and the epoxy equivalent is about 135.
example 3
[0045] Catalytic double epoxidation synthesis of example 3 CER-4221
[0046] 44g of 3-cyclohexenecarboxylic acid-3'-cyclohexene methyl ester, 2.64g of [C 5 h 5 NC 16 h 33 ] 2 [W 2 o 3 (O 2 ) 4 ], 1g sodium bicarbonate, and 88g dichloroethane were heated and stirred to 60 degrees together. 35% hydrogen peroxide (H 2 o 2 0.5mol, 2.5eq)) was added dropwise to the system, and the reaction was exothermic violently. The reaction was stirred for 1 hour, and a sample was taken for detection. The conversion rate is 99%, and the ratio of single to double is 1:17. Cool to room temperature and separate the liquids. The organic layer was washed three times with water and saturated salt, dried over anhydrous sodium sulfate, passed through a short silica gel column, and concentrated to obtain 45 g of a colorless or light yellow viscous liquid. The yield is 90%, and the epoxy equivalent is about 138.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com