Preparation method of spiromesifen
A technology of spiromethin and trimethylphenylacetyl, which is applied in the field of preparation of spiromethin, can solve the problems of unfavorable industrial production, complex and time-consuming process, and low reaction yield, so as to achieve sufficient raw material sources and simple process Effective, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
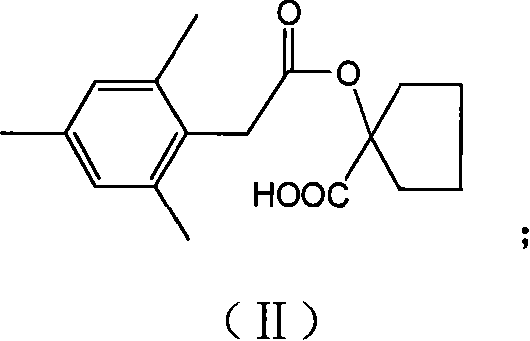
[0034] Embodiment 1, the preparation of compound II, i.e. 1-[2-(2,4,6-trimethylphenyl)-acetoxy]-cyclopentylcarboxylic acid:
[0035] Add 8.0g (61.5mmol) 1-carboxycyclopentanol, 13.5g (171mmol) pyridine, 60mL dichloromethane into the reaction flask, drop 15.6g (79.3mmol) 2,4 , The dosing of 6-trimethylphenylacetyl chloride and 40mL of dichloromethane. After the dropwise addition was completed within 1 h, the stirring reaction was continued at room temperature for 12 h.
[0036] After the reaction was completed, it was concentrated, 100 mL of 3% HCl was added, extracted three times with ethyl acetate, and washed with water. Anhydrous Na 2 SO 4 Drying and concentration yielded 15.9 g of product as a brown solid, Y=89.2%.
Embodiment 2
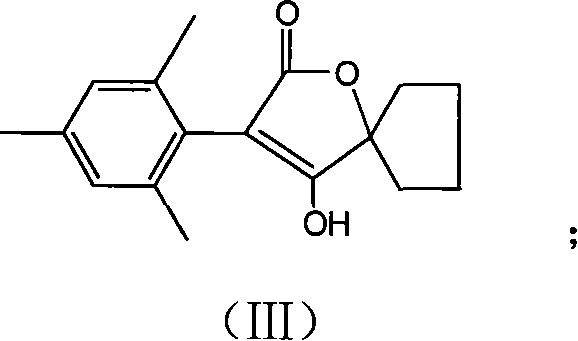
[0037] Example 2, the preparation of compound III, namely 3-(2,4,6-trimethylphenyl)-2-oxo-1-oxaspiro[4.4]-non-3-en 4-ol:
[0038] Add 10.6g (36.5mmol) 1-[2-(2,4,6-trimethylphenyl)-acetoxy]-cyclopentyl formic acid, 5.0g ( 44mmol) magnesium ethylate and 120mL of ethanol, heated to reflux for 12h.
[0039] After the reaction was over, after most of the solvent was evaporated under reduced pressure, 50 mL of 3% hydrochloric acid was added, extracted three times with ethyl acetate, and then washed with water, anhydrous Na 2 SO 4 Drying and concentration yielded 7.2 g of solid product, Y=72.5%.
Embodiment 3
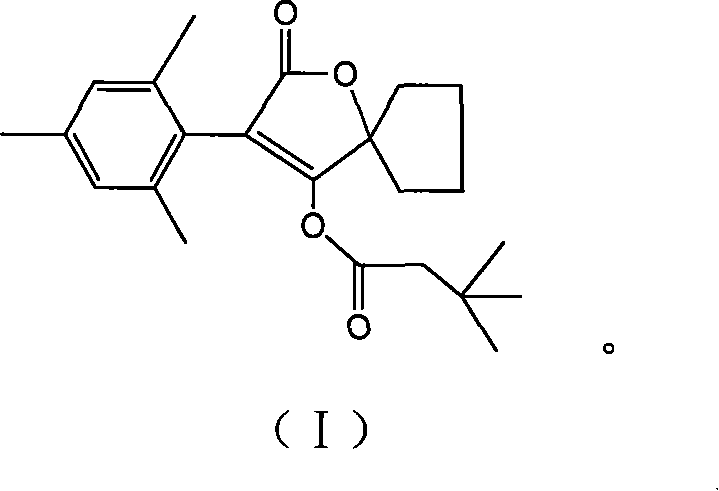
[0040] Example 3, Spiromethazol, namely 3-(2,4,6-trimethylphenyl)-2-oxo-1-oxaspiro[4.4]-non-3-en 4-yl-3 , the preparation of 3-dimethylbutyl ester:
[0041] Add 2.0 g (7.4 mmol) of 3-(2,4,6-trimethylphenyl)-2-oxo-1-oxaspiro[4.4]-nonan-3 obtained in Example 2 above to the reaction flask -en 4-alcohol and 3.0g (30.0mmol) triethylamine and 50mL dichloromethane, then add dropwise 1.3g (9.7mmol) 3,3-dimethylbutyryl chloride, and stir the reaction at room temperature (ie 0~30°C) 2h.
[0042] After the reaction, the resulting reaction solution was washed three times with 1% hydrochloric acid, and then washed with saturated NaHCO 3 The solution was washed twice, and finally washed twice with water, anhydrous Na 2 SO 4 Drying and concentration afforded 2.9 g of solid product. After recrystallization from 95% ethanol, 2.5 g of product was obtained, Y=93.1%, content 90%-95%. Finally, the product with a content of more than 99% was obtained through silica gel column purification.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com