Crystallization control and whitening control method for producing boric acid and magnesium sulfate by salt lake type solid boron ore liquid boron ore
A control method and crystallization control technology, applied in the direction of magnesium sulfate, boron oxide, etc., can solve the problems of difficult filtration, increase of solution supersaturation, large amount of washing water, etc., and achieve good washing performance, better effect and lower cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
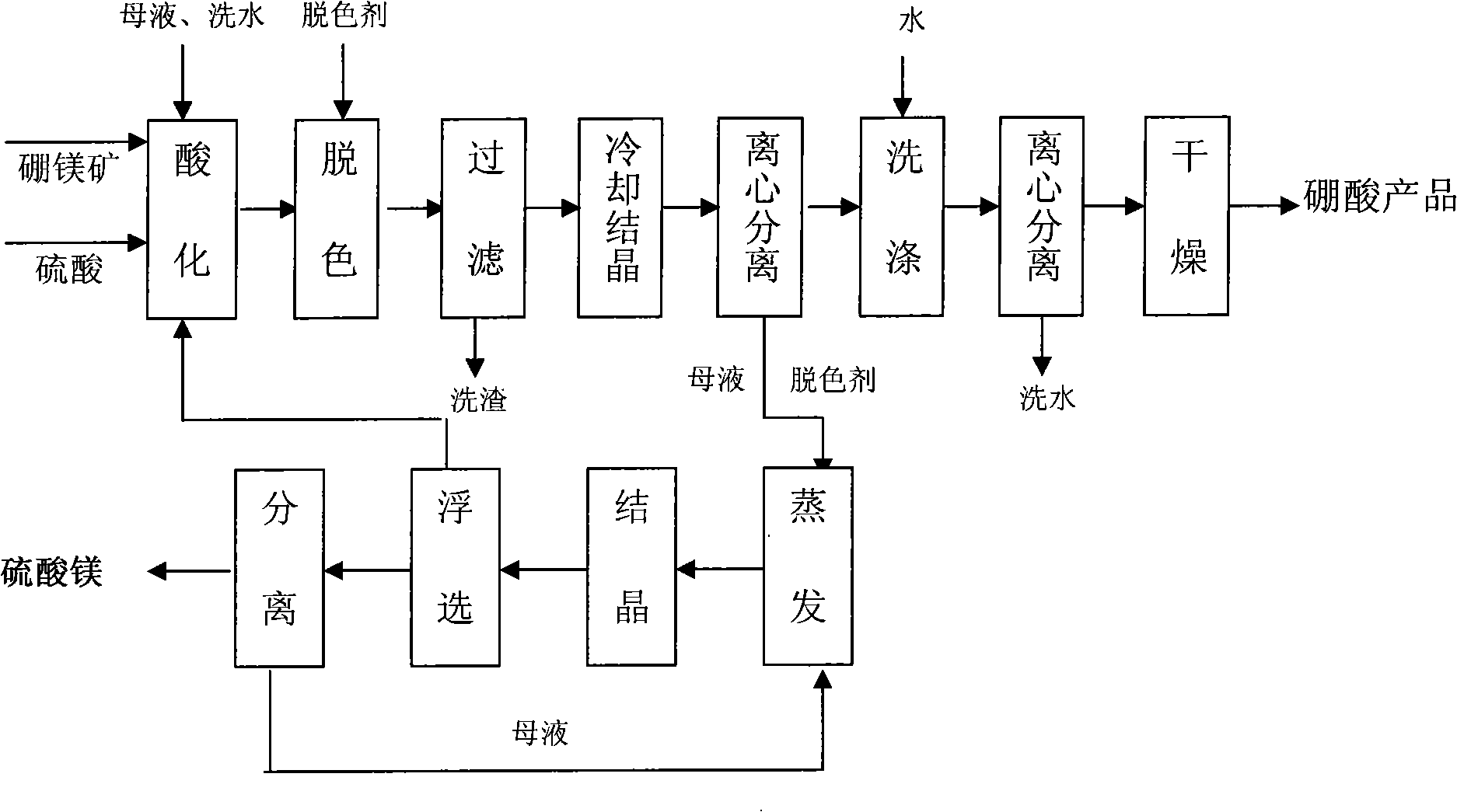
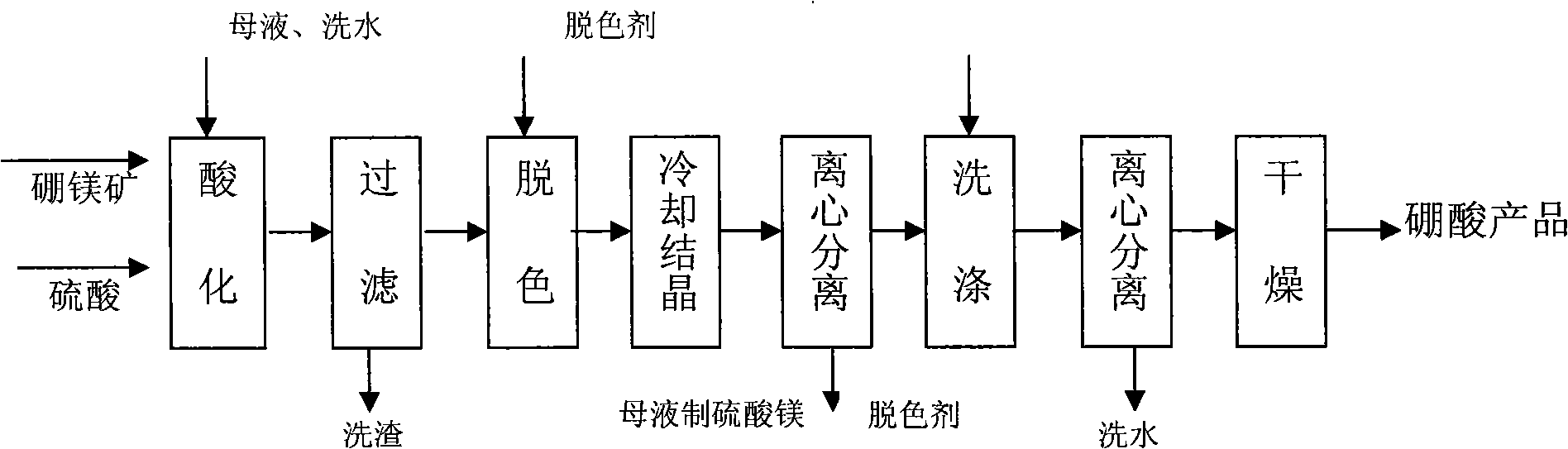
[0023] Put 9 tons of boron and magnesium ore into the acidification pot, add mother liquor, washing water and sulfuric acid to fully react, and the slurry volume is about 20 cubic meters. Hydrogen peroxide (H 2 o 2 ) according to 2.5kg / m 3 Add the amount of the mixture into the slurry and keep stirring for 30 minutes, then use a plate and frame filter press to remove the filter residue, and the filtrate is light yellow. When cooling and crystallizing, the pH of the solution is adjusted to acid repeatedly before 35°C, and the pH value is controlled between 2.8 and 4.1. When the temperature reaches 35°C, add acid to make the pH value below 2.5, and the crystals are white and coarse. Filter and wash easy. After drying, 3.69 tons of boric acid products were obtained.
[0024] Product Analysis Results: Boric Acid (H 3 BO 3 ) content % 99.50
[0025] Water insoluble content % 0.023
[0026] Sulfate (as SO 4 2- Total) content % 0.13
[0027] ...
Embodiment 2
[0031] 15 cubic acidified filtrates (reddish brown, H 3 BO 3 18% MgSO 4 27%) and hydrogen peroxide (H 2 o 2 ) according to 2.5kg / m 3 The amount was mixed, and after insulated and stirred for 30 minutes, the filtrate was light yellow. When cooling and crystallizing, the pH of the solution is adjusted to acid repeatedly before 35°C, and the pH value is controlled between 2.8 and 4.1. When the temperature reaches 35°C, add acid to make the pH value below 2.5, and the crystals are white and coarse. Filter and wash Easy, 2.2 tons of boric acid products are obtained after drying.
[0032] Product Analysis Results: Boric Acid (H 3 BO 3 ) content % 99.55
[0033] Water insoluble content % 0.024
[0034] Sulfate (as SO 4 2- Total) content % 0.15
[0035] Chloride (as Cl - Total) content % 0.068
[0036] Iron (Fe) content % 0.0020
[0037] Color: white
Embodiment 3
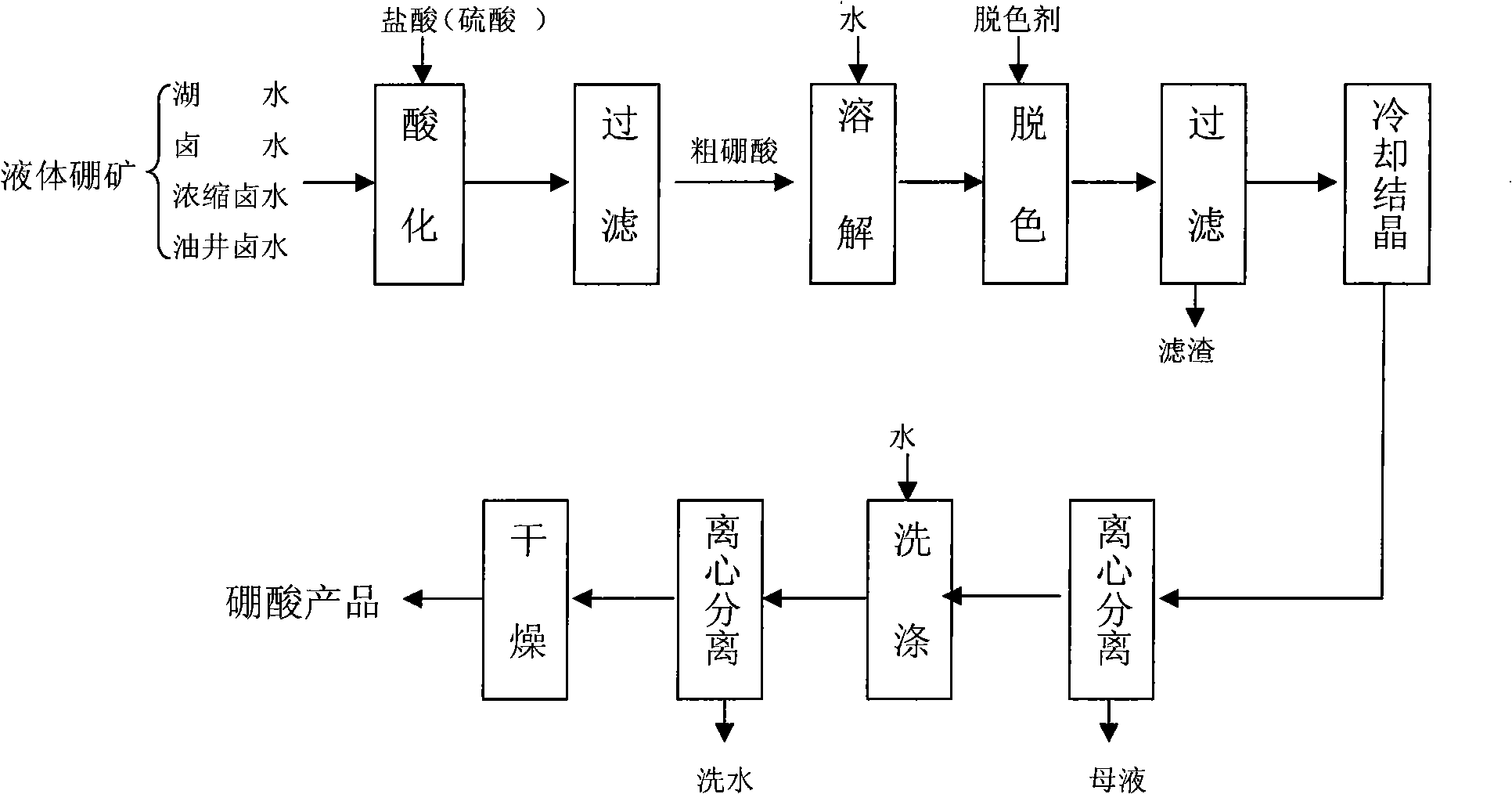
[0039] Crude boric acid is added with water to form boric acid (H 3 BO 3 ) 19% solution 15 party, the solution is yellow, hydrogen peroxide (H 2 o 2 ) according to 0.5kg / m 3 After adding, heat preservation and stirring for 30 minutes, use a plate and frame filter press to remove the filter residue, and the filtrate is light yellow. When cooling and crystallizing, the pH of the solution is adjusted to acid repeatedly before 35°C, and the pH value is controlled between 2.8 and 4.1. When the temperature reaches 35°C, add acid to make the pH value below 2.5, and the crystals are white and coarse. Filter and wash Easy, 2.3 tons of boric acid products are obtained after drying.
[0040] Product Analysis Results: Boric Acid (H 3 BO 3 ) content % 99.55
[0041] Water insoluble content % 0.015
[0042] Sulfate (as SO 4 2- Total) content % 0.13
[0043] Chloride (as Cl - Total) content % 0.060
[0044] Iron (Fe) conten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com