Secnidazole water-soluble salt and preparation method thereof
A technology of secnidazole phosphate and secnidazole, which is applied in the field of modified chemicals and their modification, and can solve the problems of inconvenient production, storage and transportation, long time, large amount of solvent and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
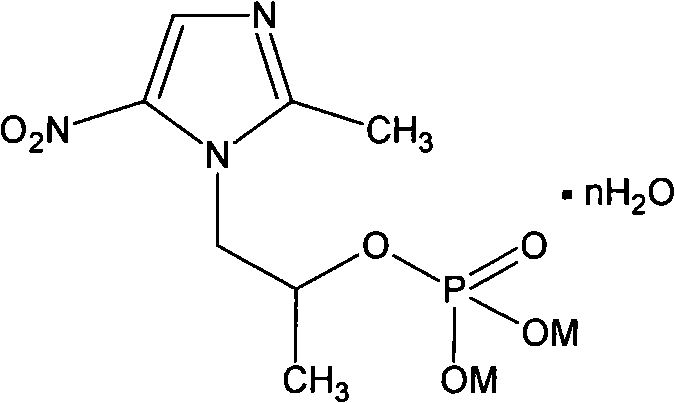
[0027] The preparation of embodiment 1 secnidazole phosphate disodium salt
[0028] Dissolve 4.6g of phosphorus oxychloride in 60mL of DMC at room temperature, put it into a three-necked flask, add 5.5g of secnidazole several times under stirring, stir for 2 hours after the addition, and precipitate a large amount of off-white solid. Raise the temperature to reflux and stir the reaction for more than 10 hours until no more acid gas is released. The liquid was poured out and distilled to dryness under reduced pressure to obtain a red solid residue. 25mL of water was added under stirring, and stirred at 50°C for 1h for hydrolysis. Activated carbon decolorizes and filters, and concentrates to dryness under reduced pressure below 60°C to obtain a reddish-brown clear transparent oil (solid after cooling), which mainly contains secnidazole phosphate. Add an appropriate amount of 90% methanol to fully dissolve, add a slight excess of sodium carbonate several times in a 40°C water b...
Embodiment 2
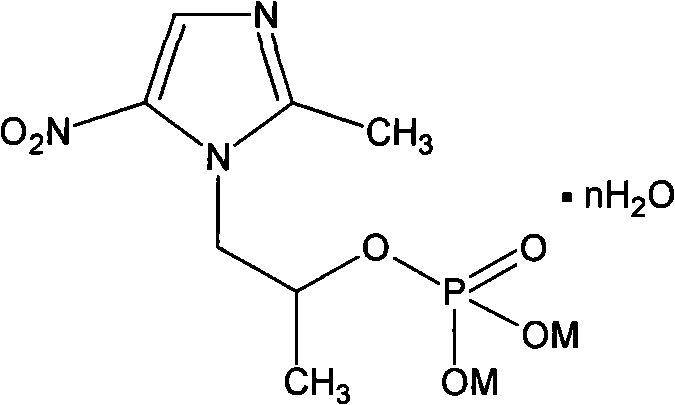
[0030] The preparation of embodiment 2 secnidazole dipotassium phosphate
[0031] The same operation as in Example 1, but sodium carbonate was changed to potassium carbonate, and the product was vacuum-dried at 160° C., and 5.5 g of secnidazole was added to obtain 5.6 g of anhydrous secnidazole dipotassium phosphate.
Embodiment 3
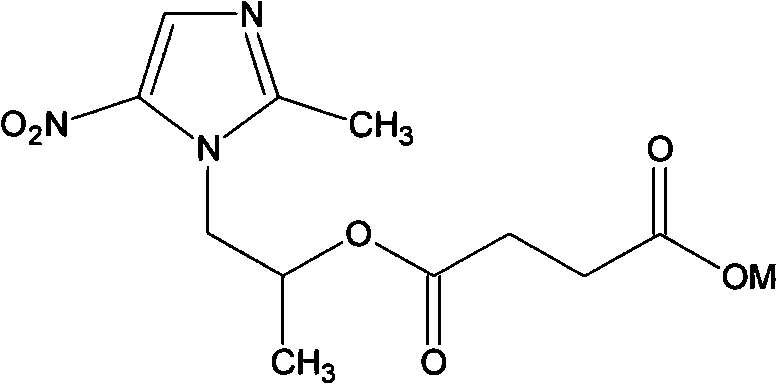
[0032] Embodiment 3 secnidazole succinic acid monoester
[0033] Cyclohexane 50ml, succinic anhydride 2.5g and pyridine 0.1mL, mix well and heat to 70°C; add 4.625g secnidazole. Reflux for more than 18 hours. Cool the reaction solution to room temperature, thick solids precipitate out at the bottom of the flask, pour out the upper liquid, add an appropriate amount of ether / carbon disulfide (4:1) to the flask, heat and stir to precipitate solids, filter, and dry to obtain secnidazole amber Acid monoester 5.2g, melting point 103-105°C. The solid is soluble in water and ethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com