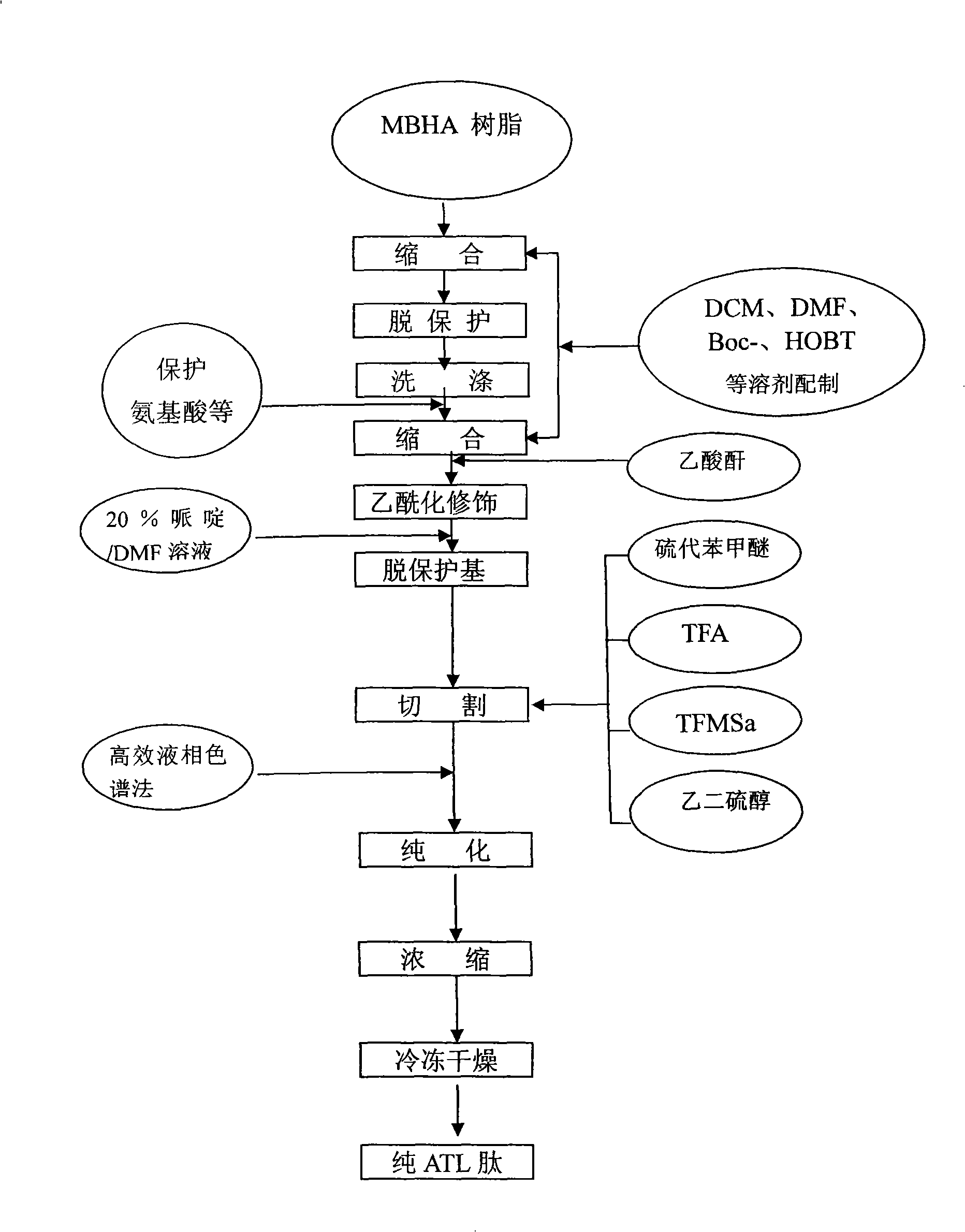
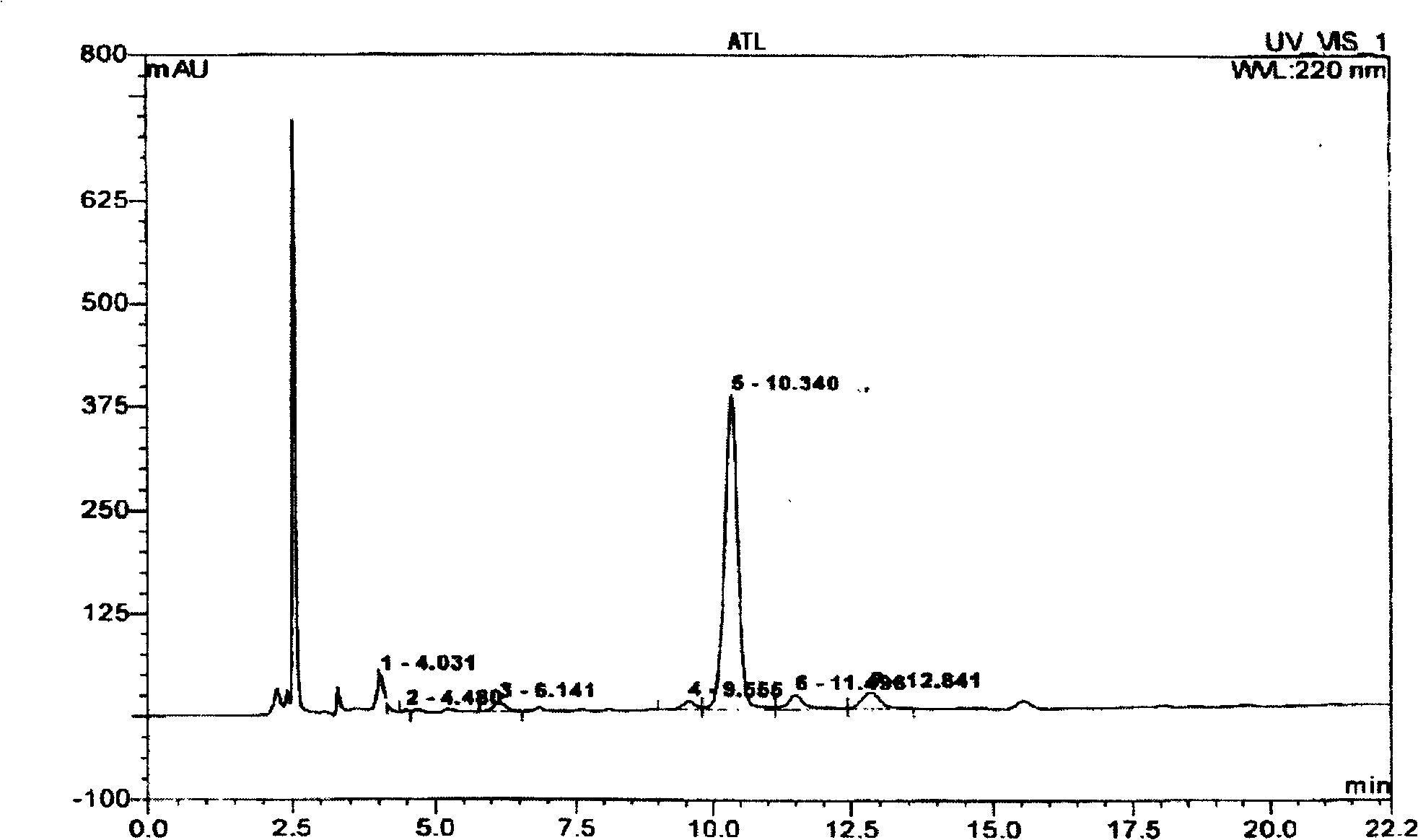
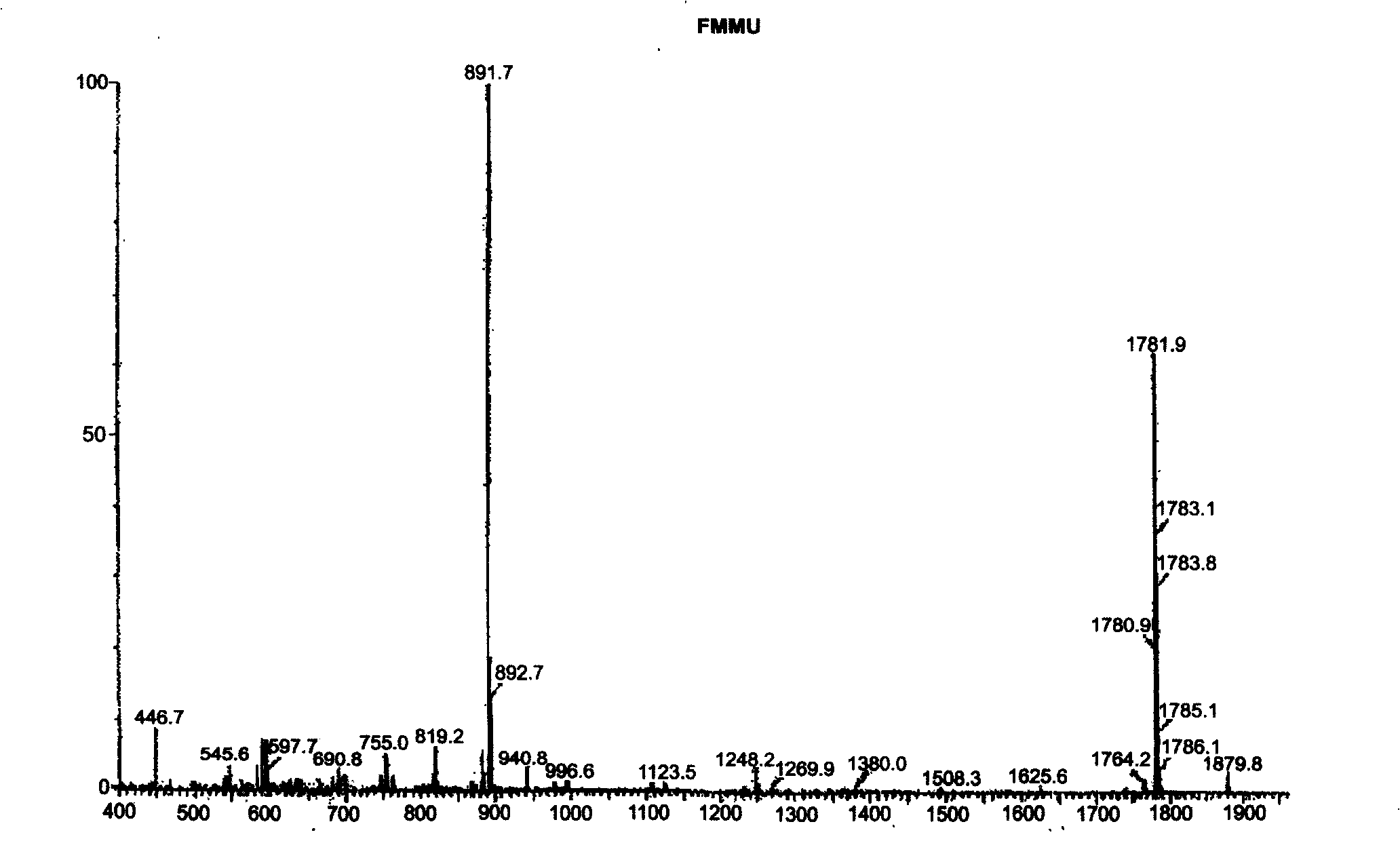
Solid-phase synthesis of ATL peptides
A technology of solid-phase synthesis and solid-phase carrier, which is applied in peptide preparation methods, chemical instruments and methods, peptides, etc., and can solve problems that are not suitable for large-scale preparation and industrial production, production scale limitations, and sources of amino acid side chain protecting groups. and difficulty in preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] h 2 Preparation of N-Lys(2-Cl-Z)-MBHA
[0043] (1) Put 50g (40mmol) of MBHA resin in a polypeptide synthesis reactor, wash it twice with 5% triethylamine DCM solution 5-7ml / g, each time for 8 minutes; alternately wash with 300ml each of dichloromethane and ethanol 2 times, 2 minutes / time, drain.
[0044] (2) Mix Boc-Lys(2-Cl-Z)-OH 82.8g and HOBt 27.0g, N,N-cyclohexylcarbodiimide 41.2g with 500ml dichloromethane / DMF solution (dichloromethane:N, N-dimethylformamide (volume ratio: 1:0.8) was dissolved, added to the resin, stirred at room temperature for 5 hours, and drained.
[0045] (3) The product of (2) was alternately washed 3 times with 300 ml of dichloromethane and 300 ml of ethanol, each time for 2 minutes, and drained.
[0046] (4) Wash once with 50% trifluoroacetic acid / dichloromethane (DCM) 10ml / g for 20 minutes; wash twice with dichloromethane 5-7ml / g for 2 minutes each time; get: H 2 N-Lys(2-Cl-Z)-MBHA.
Embodiment 2
[0048] h 2 Preparation of N-Lys(2-Cl-Z)-Lys(2-Cl-Z)-MBHA
[0049] (1) to resin H 2 Add 5% triethylamine DCM solution 5-7ml / g to N-Lys(2-Cl-Z)-MBHA and wash twice, 8 minutes each time; alternately wash twice with 300ml dichloromethane and ethanol, 2 minutes / times, drained.
[0050] (2) Mix Boc-Lys(2-Cl-Z)-OH 82.8g and HOBt 27.0g, N,N-cyclohexylcarbodiimide 41.2g with 500ml dichloromethane / DMF solution (dichloromethane:N, N-dimethylformamide (volume ratio: 1:0.9) was dissolved, added to the resin, stirred at room temperature for 5 hours, and drained.
[0051] (3) The product of (2) was alternately washed 3 times with 300 ml of dichloromethane and 300 ml of ethanol, each time for 2 minutes, and drained.
[0052] (4) Wash once with 50% trifluoroacetic acid / dichloromethane (DCM) 10ml / g for 20 minutes;
[0053] Wash twice with dichloromethane 5-7ml / g, 2 minutes each time;
[0054] Got: H 2 N-Lys(2-Cl-Z)-Lys(2-Cl-Z)-MBHA.
Embodiment 3
[0056] h 2 The preparation operation of N-Lys(2-Cl-Z)-Lys(2-Cl-Z)-Lys(2-Cl-Z)-MBHA: (1) to resin H 2 Add 5% triethylamine DCM solution 5-7ml / g to N-Lys(2-Cl-Z)-Lys(2-Cl-Z)-MBHA and wash twice, each time for 8 minutes; Each 300ml was alternately washed twice, 2 minutes each time, and drained.
[0057] (2) Mix Boc-Lys(2-Cl-Z)-OH 82.8g and HOBt 27.0g, N,N-cyclohexylcarbodiimide 41.2g with 500ml dichloromethane / DMF (dichloromethane:N,N - dimethylformamide (volume ratio: 1:0.9) solution was dissolved, added to the resin, stirred at room temperature for 5 hours, and drained.
[0058] (3) The product of (2) was alternately washed 3 times with 300 ml of dichloromethane and 300 ml of ethanol, each time for 2 minutes, and drained.
[0059] (4) Wash once with 50% trifluoroacetic acid / dichloromethane (DCM) 10ml / g for 20 minutes;
[0060] Wash twice with dichloromethane 5-7ml / g, 2 minutes each time;
[0061] Got: H 2 N-Lys(2-Cl-Z)-Lys(2-Cl-Z)-Lys(2-Cl-Z)-MBHA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com