Method for preparing adriamycin-dipeptide complexes and applications
A technology of peptide complexes and doxorubicin, which is applied in medical preparations with non-active ingredients, polymer compounds with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low reaction rate, long time consumption, and product production. To solve the problems of low efficiency and purity, achieve the effect of high reaction rate, short time consumption, high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of benzyloxycarbonylglycylprolyl doxorubicin
[0028] (1) Activation of N-benzyloxycarbonylglycylproline:
[0029] Weigh 26.4mg (86.2μmol) of N-benzyloxycarbonylglycylproline and dissolve it in 1.5ml of N,N-dimethylformamide (DMF), add 9.8μl (86.2×1.2=103.4μmol) of chlorine Ethyl formate, stirred at room temperature for 10 minutes, added 14.5 μl (103.4 μmol) of triethylamine, stirred at room temperature for 40 minutes.
[0030] (2) Preparation of N-benzyloxycarbonylglycylprolyl doxorubicin:
[0031] Weigh 40 mg (86.2 μmol) of doxorubicin hydrochloride and dissolve it in 1.5 mL of DMF, add 12.5 μl (86.2 μmol) of triethylamine and mix well. Add the dissolved doxorubicin hydrochloride solution to the above-mentioned activated Z-GP solution, and stir at room temperature for 5 to 6 hours; the product is extracted with 30 ml of ether (5 times the volume of the reaction solution), and the precipitate is discarded by suction filtration under reduced pressu...
Embodiment 2
[0034] Example 2 In vitro cytotoxicity test of benzyloxycarbonylglycylprolyl doxorubicin (Z-GP-Dox)
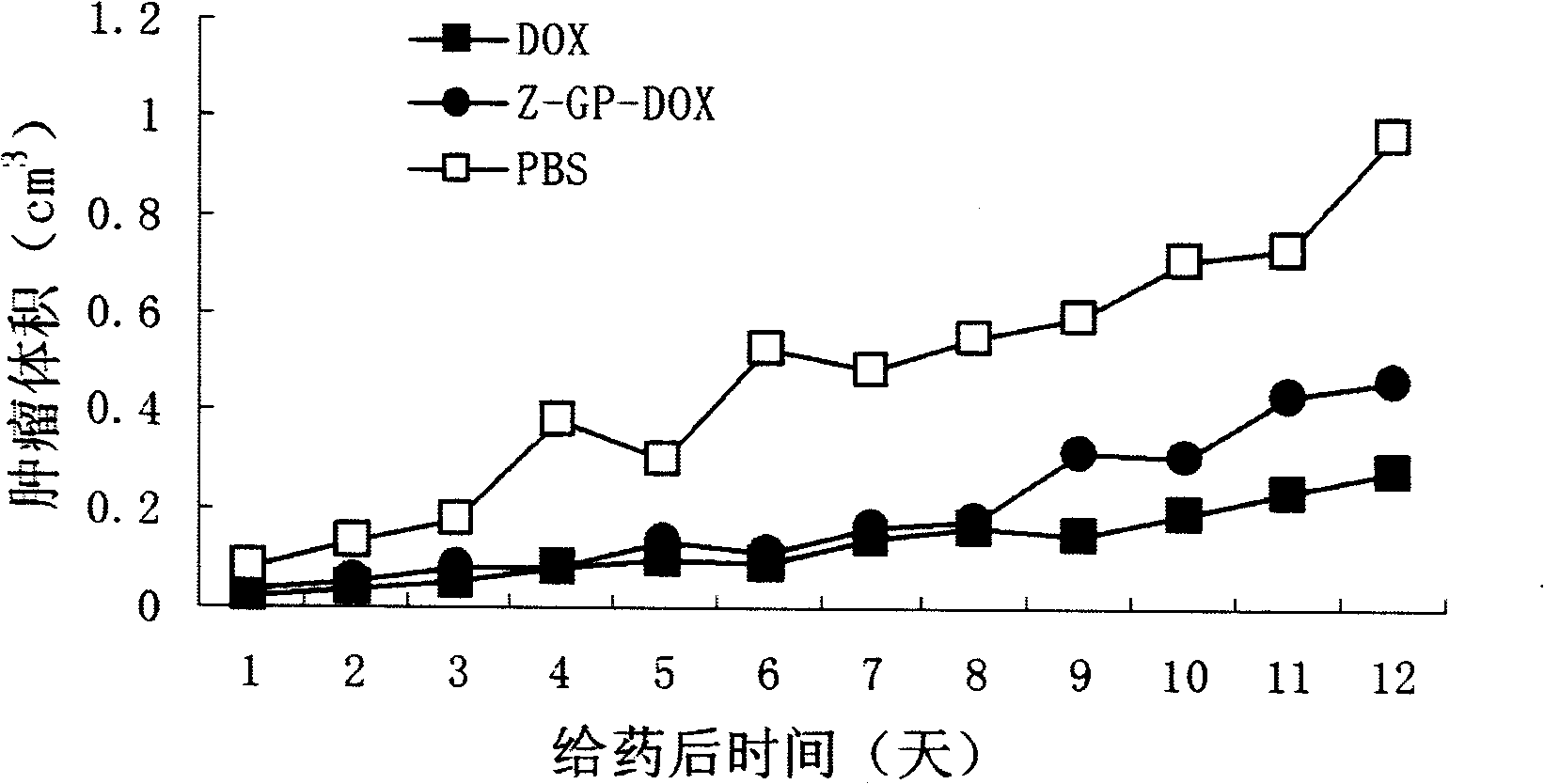
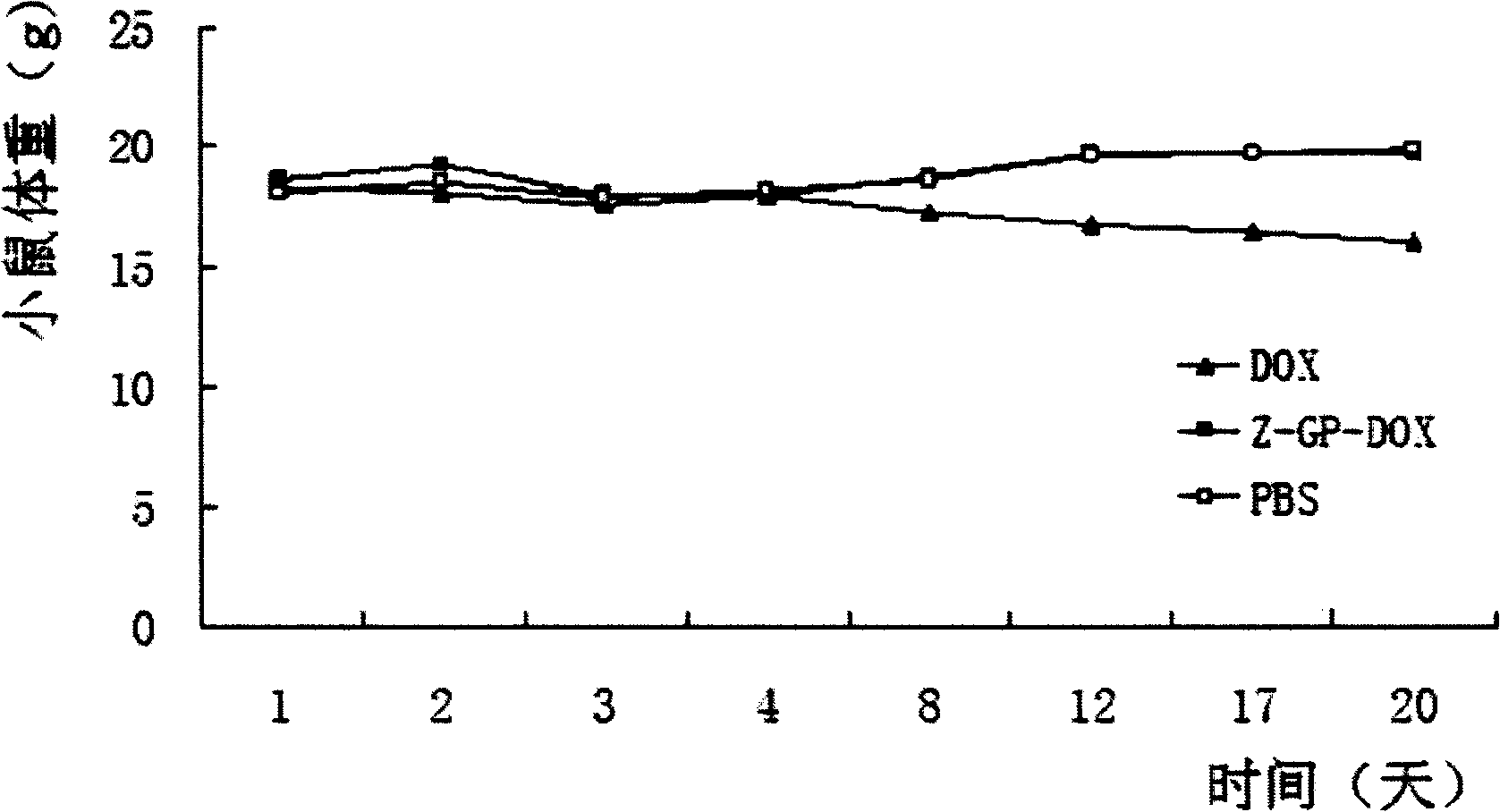
[0035] K562, MOLT-4 cells were seeded in 96-well plates at a density of 10,000 / well, and free doxorubicin containing 0 μM-2 μM or Z-GP-Dox at an equimolar concentration (the solvent was DMSO) was added to the cells, Or replace the K562, MOLT-4 cell culture medium with the mEF cell culture medium incubated with the Z-GP-Dox of the above-mentioned equimolar concentration of free doxorubicin for 24 hours and 48 hours, after 24 hours and 48 hours, respectively, by The cell viability of K562 and MOLT-4 was detected by MTT method. From the results, the cytotoxicity of Z-GP-Dox was reduced by 4-7 times (K562 cells) and 9-24 times (MOLT-4 cells) respectively compared with the IC50 of Dox. However, after incubation with mEF cells, Z-GP-Dox can recover to the cytotoxicity IC50 similar to that of the parent drug, as shown in Table 1.
[0036] Table 1 Cytotoxicity comparison of Z-GP-Dox...
Embodiment 3
[0038] Example 3 Plasma stability test of benzyloxycarbonylglycylprolyl doxorubicin (Z-GP-Dox)
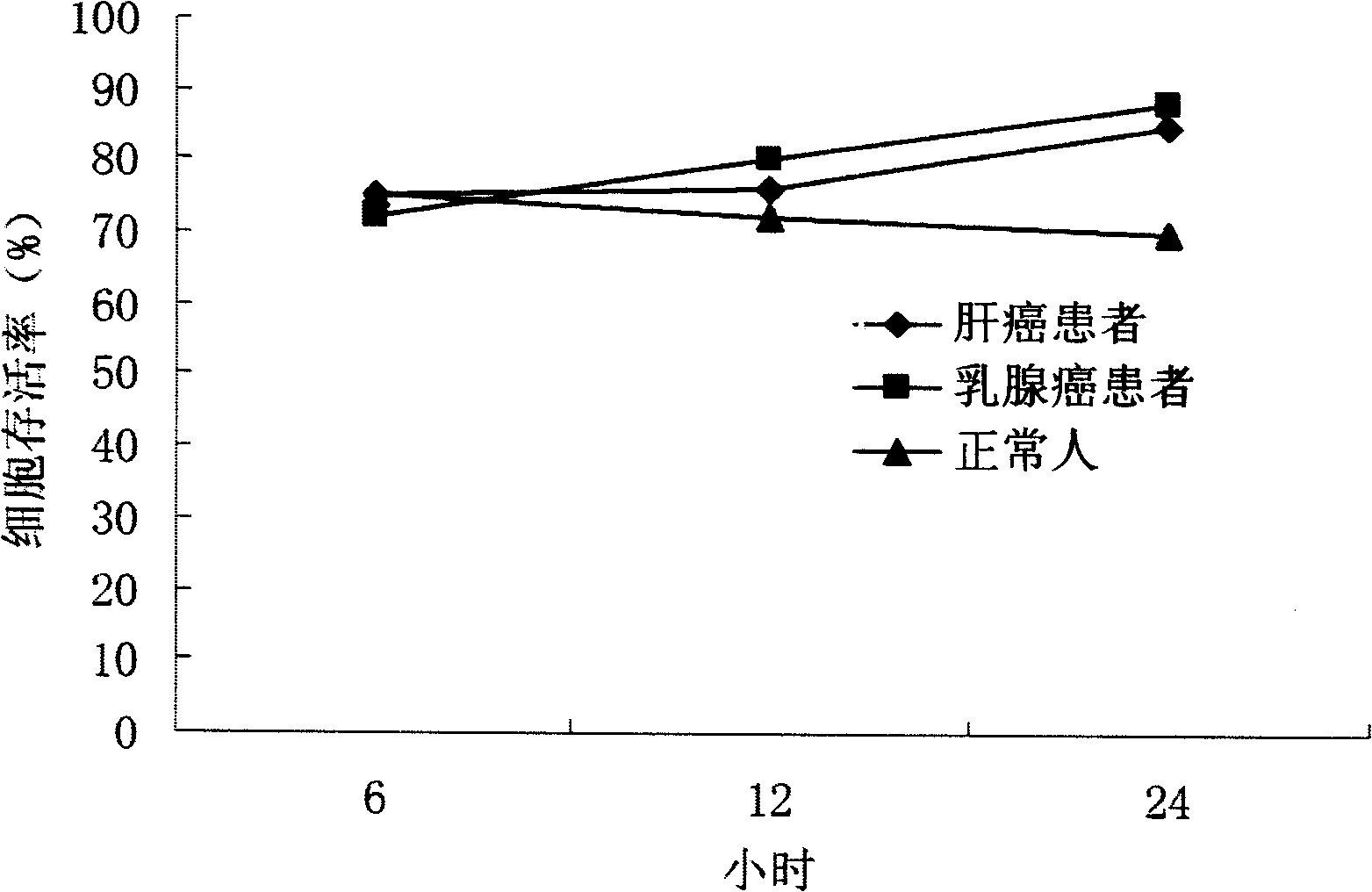
[0039] Containing 0.675μM Z-GP-Dox in 20% liver cancer cells, breast cancer patient serum and normal human plasma for 6 hours, 12 hours, and 24 hours respectively, the supernatant was used to act on K562 cells, and the cell survival rate was detected by MTT method. The results showed that after 24 hours, the cell survival rate still reached more than 70%, indicating that Z-GP-Dox was less cleaved into the cytotoxic parent drug doxorubicin in the serum, and it was less effective in the serum of patients with liver cancer, breast cancer patients and normal people. in is stable. See attached figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com