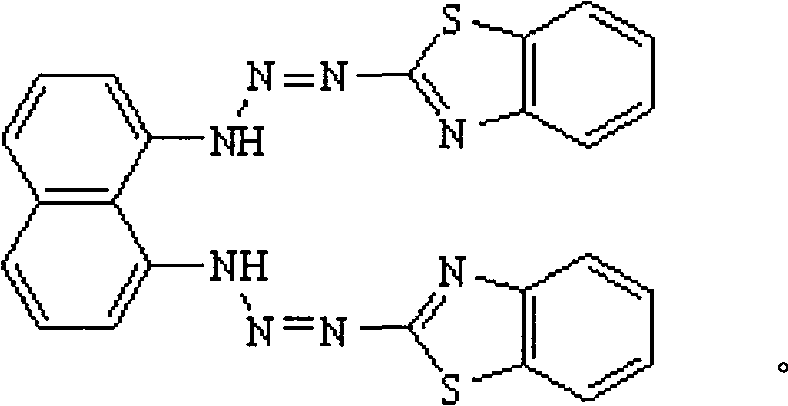
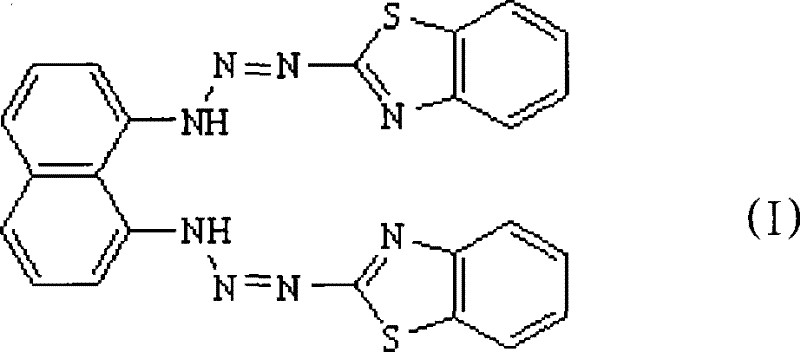
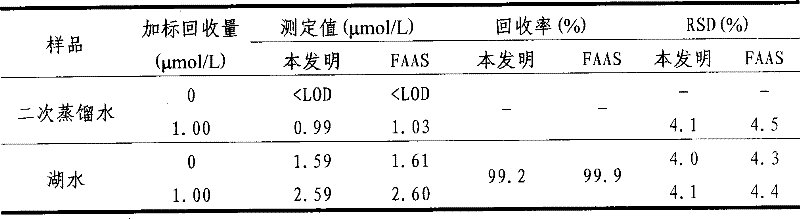
1,8-bis(2-benzothiazolydiazoamino)- naphthaline, preparation and application thereof
A diazoamino and benzothiazole technology, which is applied in the direction of chemical reaction of materials for analysis, fluorescence/phosphorescence, chemiluminescence/bioluminescence, etc., can solve the problems of triazene reagent interference and achieve high sensitivity, The preparation method is simple and the selectivity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1), the diazotization of 2-aminobenzothiazole
[0024] Add 1mL of formic acid, 3mL of water, 2mL of concentrated sulfuric acid and 0.6g (0.004mol) of 2-aminobenzothiazole to the flask in sequence, slowly add 2mL of 0.14g / mL sodium nitrite aqueous solution under stirring at 3°C, and react for 30min to make the diazo complete.
[0025] 2), the preparation of 1,8-bis(2-benzothiazolediazoamino)naphthalene
[0026] Dissolve 0.316g (0.002mol) of 1,8-naphthalenediamine in 20mL of ethanol, slowly add the above diazonium salt solution under stirring at 0°C, adjust the pH of the mixture to 6 with sodium acetate solution, react for 2 hours, and store in the refrigerator Stand overnight, filter with suction, and wash with 50% aqueous ethanol. After drying, it was applied to a chromatographic column and separated by column chromatography using ethyl acetate:petroleum ether=2:3 (volume ratio) as the eluent, and then recrystallized with 95% ethanol to obtain a black pure product.
...
Embodiment 2
[0029] 1), the diazotization of 2-aminobenzothiazole
[0030] Add 2mL of formic acid, 4mL of water, 3mL of concentrated sulfuric acid, 1mL of phosphoric acid and 0.75g (0.005mol) of 2-aminobenzothiazole in the flask, and slowly add 3mL of 0.14g / mL sodium nitrite aqueous solution at 2°C under stirring. React for 40 minutes to complete the diazotization.
[0031] 2), the preparation of 1,8-bis(2-benzothiazolediazoamino)naphthalene
[0032] Dissolve 0.316g (0.002mol) of 1,8-naphthalenediamine in 25mL of ethanol, slowly add the above diazonium salt solution under stirring at 3°C, adjust the pH of the mixture to 7 with sodium acetate solution, react for 2.5h, and Let stand overnight in the refrigerator, filter with suction, and wash with 50% aqueous ethanol. After drying, it was applied to a chromatographic column and separated by column chromatography using ethyl acetate:petroleum ether=2:3 (volume ratio) as the eluent, and then recrystallized with 95% ethanol to obtain a black ...
Embodiment 3
[0034] 1), the diazotization of 2-aminobenzothiazole
[0035] Add 2.5mL of acetic acid, 6mL of water, 4mL of concentrated sulfuric acid and 0.675g (0.0045mol) of 2-aminobenzothiazole in sequence in the flask, and slowly add 3.5mL of 0.14g / mL sodium nitrite aqueous solution at 3°C under stirring, and react 40min to complete the diazotization.
[0036] 2), the preparation of 1,8-bis(2-benzothiazolediazoamino)naphthalene
[0037] Dissolve 0.474g (0.003mol) of 1,8-naphthalenediamine in 30mL of ethanol, slowly add the above diazonium salt solution under stirring at 3°C, adjust the pH of the mixture to 5 with saturated sodium carbonate solution, and react for 1.6h. Stand overnight in the refrigerator, filter with suction, and wash with 50% aqueous ethanol. After drying, it was applied to a chromatographic column and separated by column chromatography using ethyl acetate:petroleum ether=2:3 (volume ratio) as the eluent, and then recrystallized with 90% ethanol to obtain a black p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com