Preparation for high-carboxyl-content oxalic acid starch
A high carboxyl and starch technology, applied in the field of carboxyl starch, can solve the problems of limiting the wide application of carboxyl starch, low carboxyl content, low oxidation degree of products, etc., and achieve the effect of easy industrial application, high degree of substitution, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
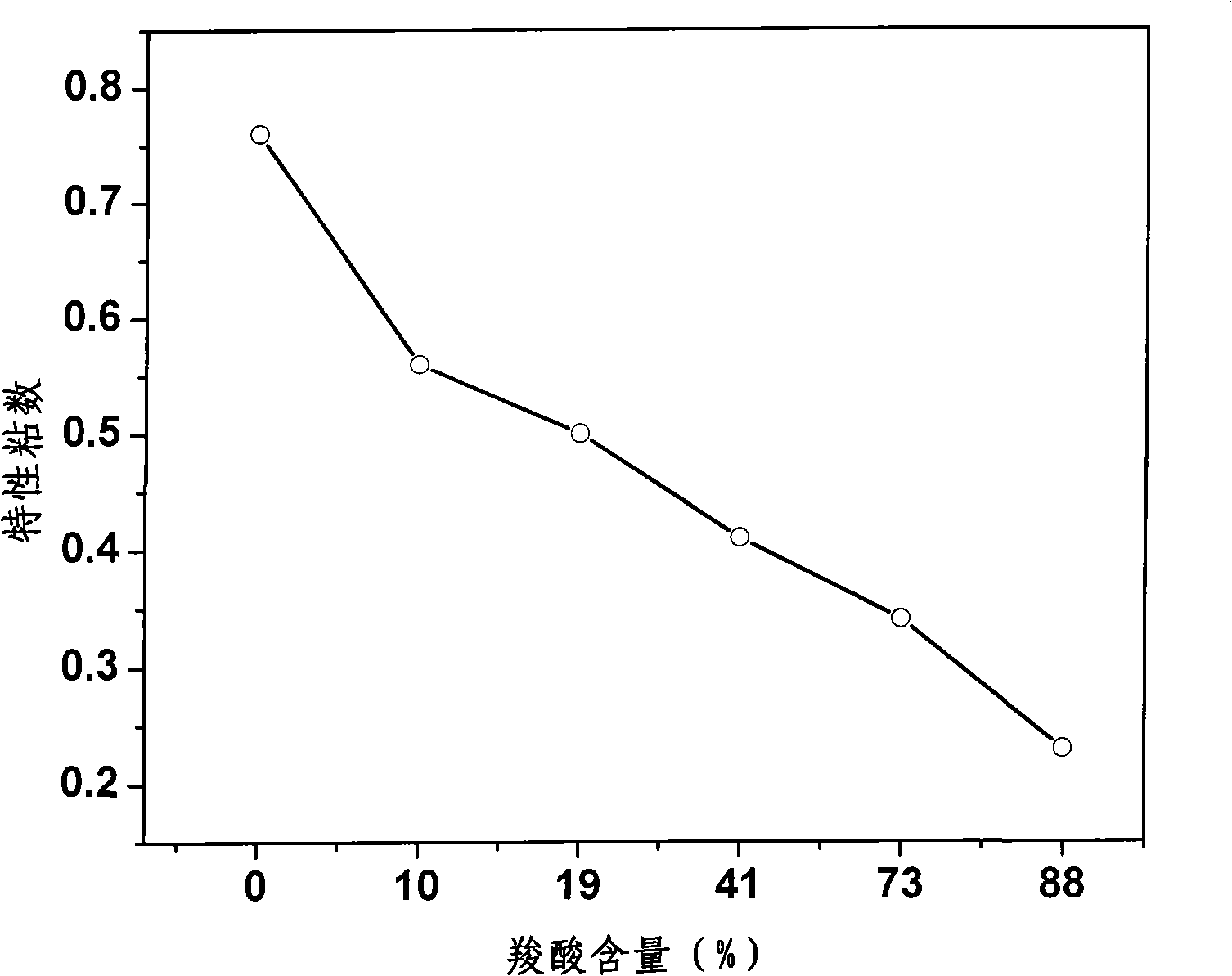
[0034]Weigh 50 grams of oxalic acid and dissolve it in 50 milliliters of dimethyl sulfoxide. After the oxalic acid is completely dissolved, move it to a ground flask, add 10 grams of dry starch with a particle size of 50 mesh, and then add 100 milliliters of benzene The solution was mixed with toluene (2:1, volume ratio), and 0.1 ml of pyridine was used as a catalyst for the reaction. Connect a water separator and a condenser, and react with magnetic stirring at a temperature of 100° C. for 18 hours. After the reaction, cool down, add 100 milliliters of ethanol and stir to obtain carboxyl starch precipitate, and the precipitate obtained after suction filtration is washed with 50% ethanol until the pH of the filtrate is 7. The obtained solid was dried at 95° C. for 24 hours, and its carboxyl content was measured to be 88%, with a yield of 85.3%.
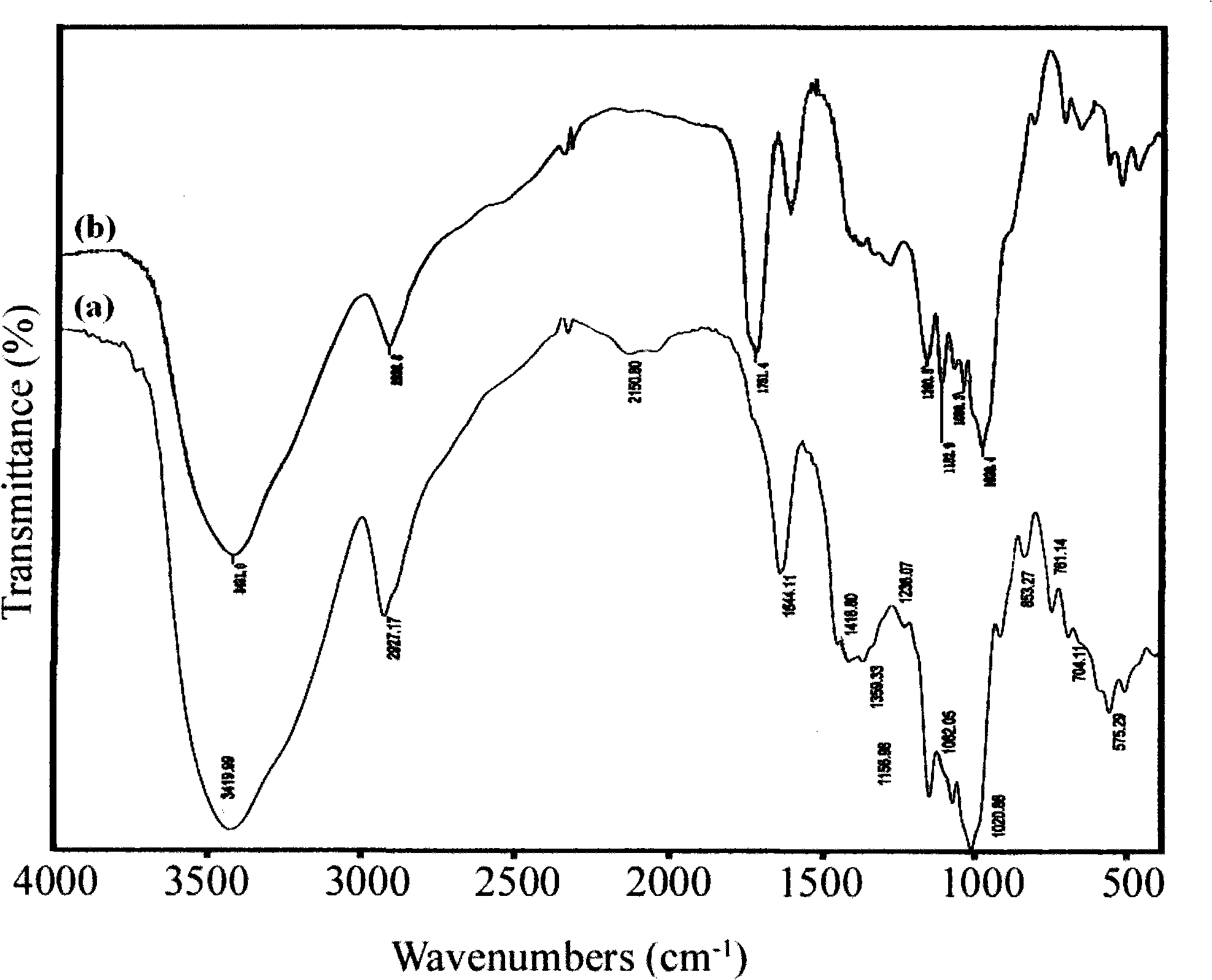
[0035] figure 1 Be the infrared spectrogram of embodiment 1 gained oxalate starch; Wherein (a): starch; (b): oxalate starch. It c...
Embodiment 2
[0038] Weigh 50 grams of oxalic acid and dissolve it in 300 milliliters of dimethylformamide. After the oxalic acid is completely dissolved, move it to a ground flask, add 300 grams of dry starch with a particle size of 200 mesh, and then add 300 milliliters of benzene , 3 mL of pyridine was used as a catalyst for the reaction. Connect a water separator and a condenser, and react with magnetic stirring at a temperature of 100° C. for 72 hours. After the reaction, cool down, add 100 milliliters of ethanol and stir to obtain carboxyl starch precipitate, and the precipitate obtained after suction filtration is washed with 50% ethanol until the pH of the filtrate is 7. The obtained solid was dried at 90° C. for 24 hours, and its carboxyl content was measured to be 10.0%, with a yield of 83.5%.
Embodiment 3
[0040] Weigh 50 grams of oxalic acid and dissolve it in 150 milliliters of dimethylacetamide. After the oxalic acid is completely dissolved, move it to a ground flask, add 150 grams of dry starch with a particle size of 100 mesh, and then add 200 milliliters of toluene , 2 ml of concentrated sulfuric acid was used as a catalyst for the reaction. Connect a water separator and a condenser, and react with magnetic stirring at a temperature of 100° C. for 48 hours. After the reaction, cool down, add 100 milliliters of ethanol and stir to obtain carboxyl starch precipitate, and the precipitate obtained after suction filtration is washed with 50% ethanol until the pH of the filtrate is 7. The obtained solid was dried at 100° C. for 18 hours, and its carboxyl content was measured to be 19%, with a yield of 84.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com