Phosphonated rifamycins and uses thereof for the prevention and treatment of bone and joint infections
一种利福霉素、膦酸化的技术,应用在化学仪器和方法、非有效成分的医用配制品、含有效成分的医用配制品等方向,能够解决骨特异性递送骨骼独特解剖学特性限制、没有显示出靶向骨骼活性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0448] Example 1 : Synthesis of Phosphonated Rifamycins
[0449] A) General experimental method
[0450] A1) Preparation of bisphosphonate structural unit
[0451]
[0452] Tetraesters (I) of methylene diphosphonic acid can be prepared from the parent tetrachloride and an alcohol as described in Synth. Commun. (2002), 32; 211-218.
[0453]
[0454] Alkylation of the anion of I with 4-substituted benzyl bromide II or bromoacetate IV following the protocol described in Bioorg. Med. Chem. (1999), 7; 901-919 affords general formulas III and V The benzyl-substituted bisphosphonate building block. Under hydrogenation conditions, with a catalyst such as PtO 2 Reduction of the nitro group converts the nitro compound IIIa to the aniline IIIb. Esters such as IIIc and Va can be converted to the corresponding acids IIId or Vb by ester cleavage. For example, an ester IIIc of R'=t-Bu can be treated with TFA to give the corresponding acid IIId. Under similar conditions, the e...
Embodiment 2
[0633] Example 2 : Determination of Antibacterial Activity and Cytotoxicity in Vitro
[0634] In vitro antibacterial activity
[0635] The susceptibility of S. aureus strains to known antibiotics and synthetic compounds was determined following the guidelines proposed by NCCLS (M26-A). Compounds were serially diluted 2-fold in DMSO and transferred to cation-adjusted Mueller Hinton broth (CAMHB; Becton Dickinson). Mix 50 μl of compound diluted in CAMHB with 100 μl of bacteria diluted in CAMHB in a 96-well microtiter plate. The final concentration of microorganisms in the assay was 5x10 -5 c.f.u. / ml, while the final concentration of DMSO in the assay was 1.25%. Assays were performed in duplicate and incubated at 37°C for 18 hours. The lowest concentration of a compound reported to inhibit visible growth is the minimum inhibitory concentration (MIC).
[0636] Sensitivity assay experiments were also performed in the presence of serum. These experiments were performed in a...
Embodiment 3
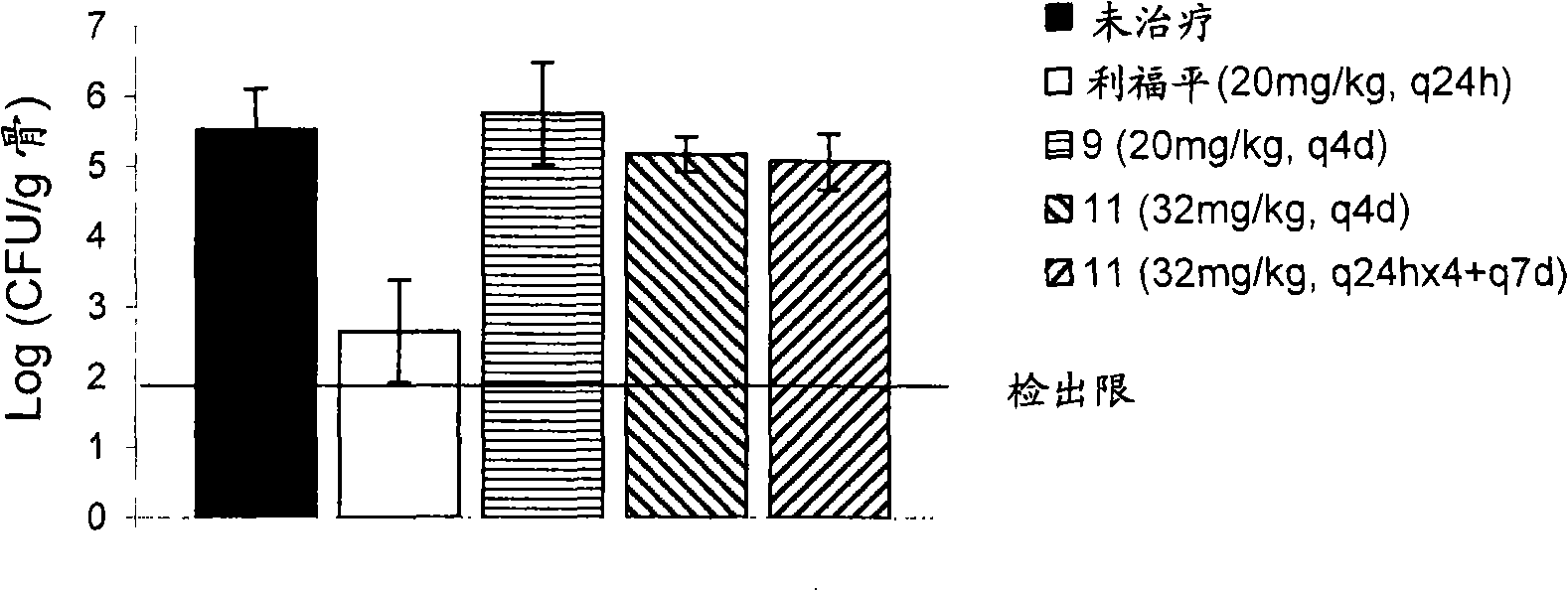
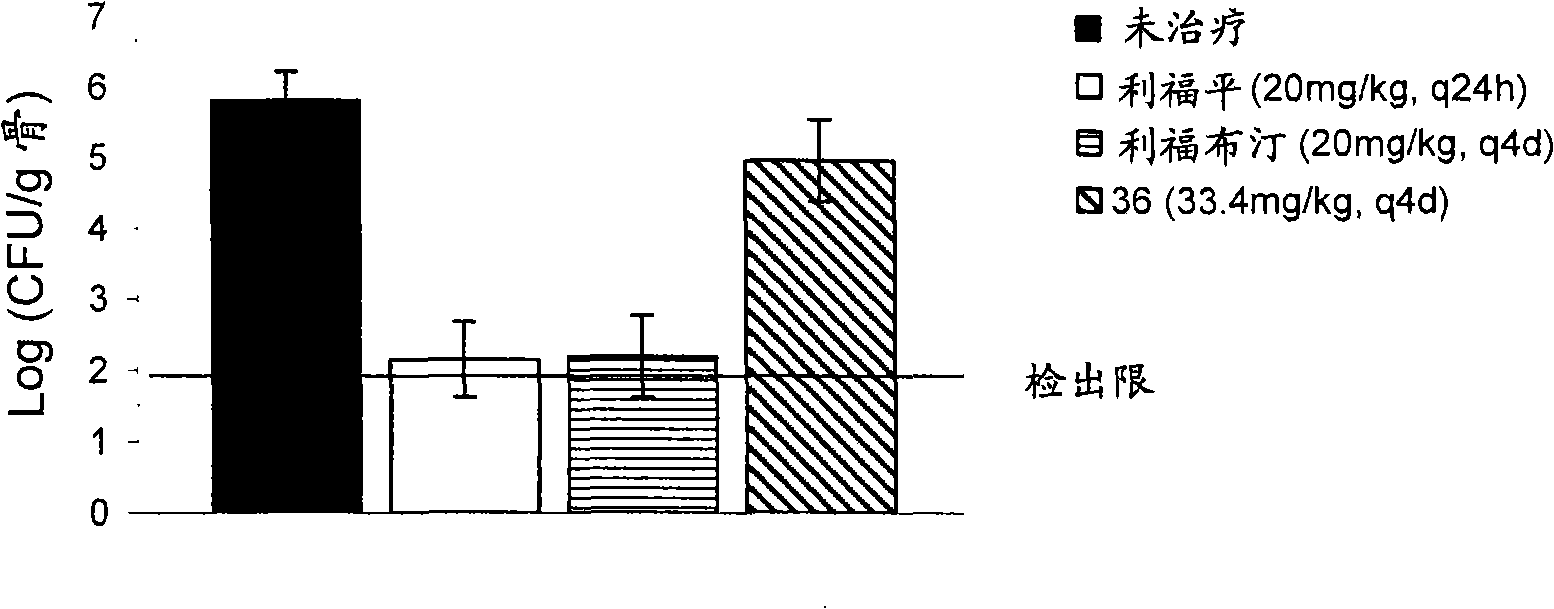
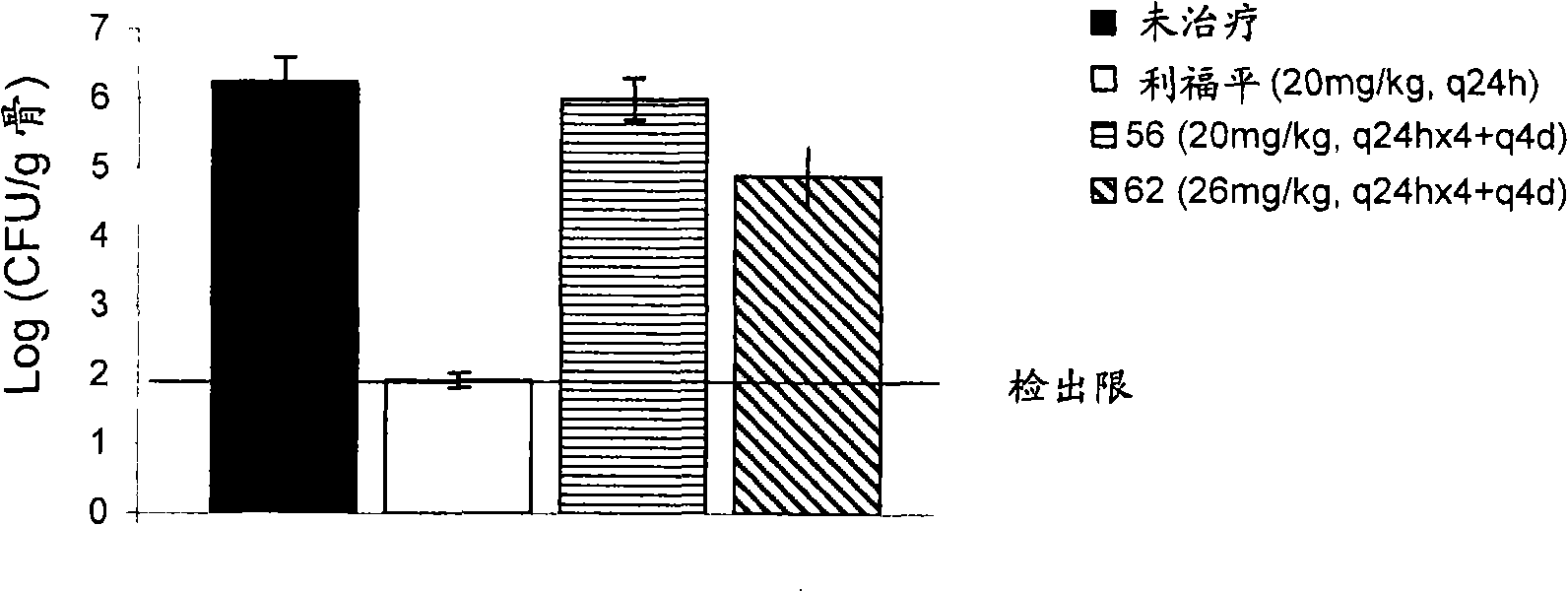
[0642] Example 3 : In vitro conjugation of compounds to bone powder and subsequent regenerated bone powder conjugation of the parent drug
[0643] The ability of the molecule of Example 1 to bind bone meal was determined using microbiological assays. Each compound was dissolved in PBS and resuspended at a concentration of 1 mg / ml in PBS of a 10 mg / ml bone meal (Now Foods, Bloomingdale, Illinois, USA) slurry. The suspension of drug / prodrug and bone powder was incubated at 37°C for 1 hour to allow it to bind, centrifuged at 13000 rpm for 2 minutes, and the supernatant was recovered. The bone powder pellet was washed three times with 1 ml PBS+2% DMSO. The content of the prodrug in the supernatant was detected by the following microbiological assay: Colonies isolated from the indicator strain (S. aureus K1758) were resuspended in 0.85% saline until OD600 = 0.1 on cation-adjusted Miller Hinton agar (CAMHA) draw a line on the board. Add known volumes of supernatant to small dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com