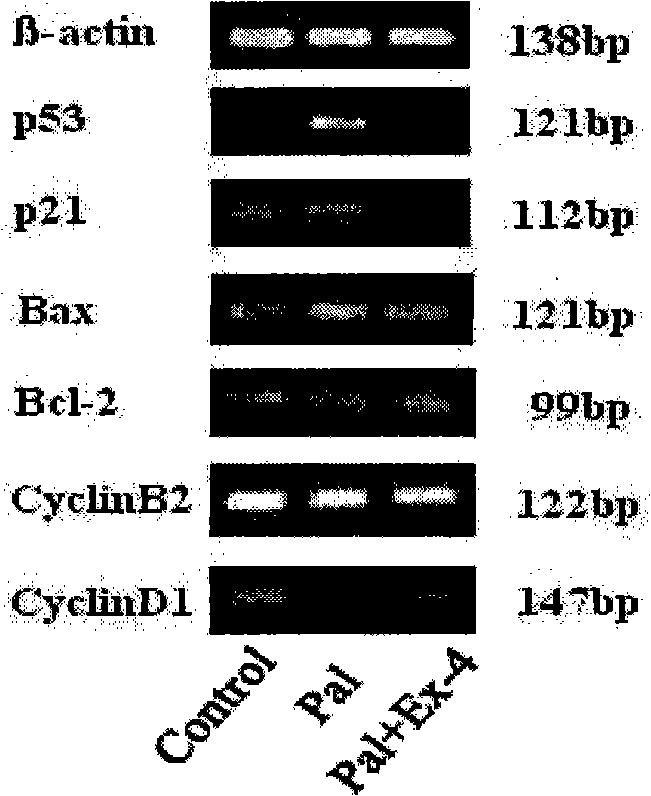
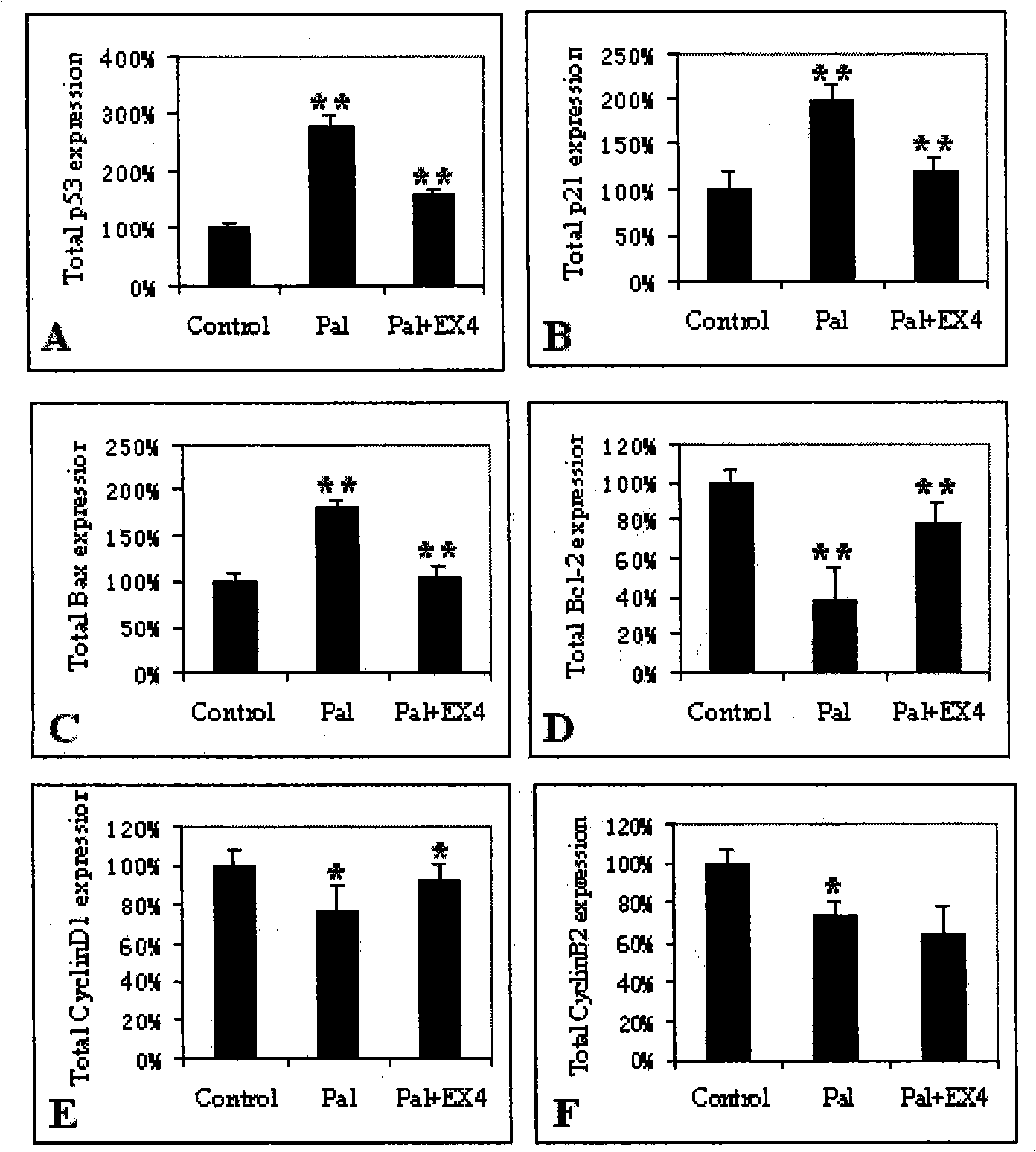
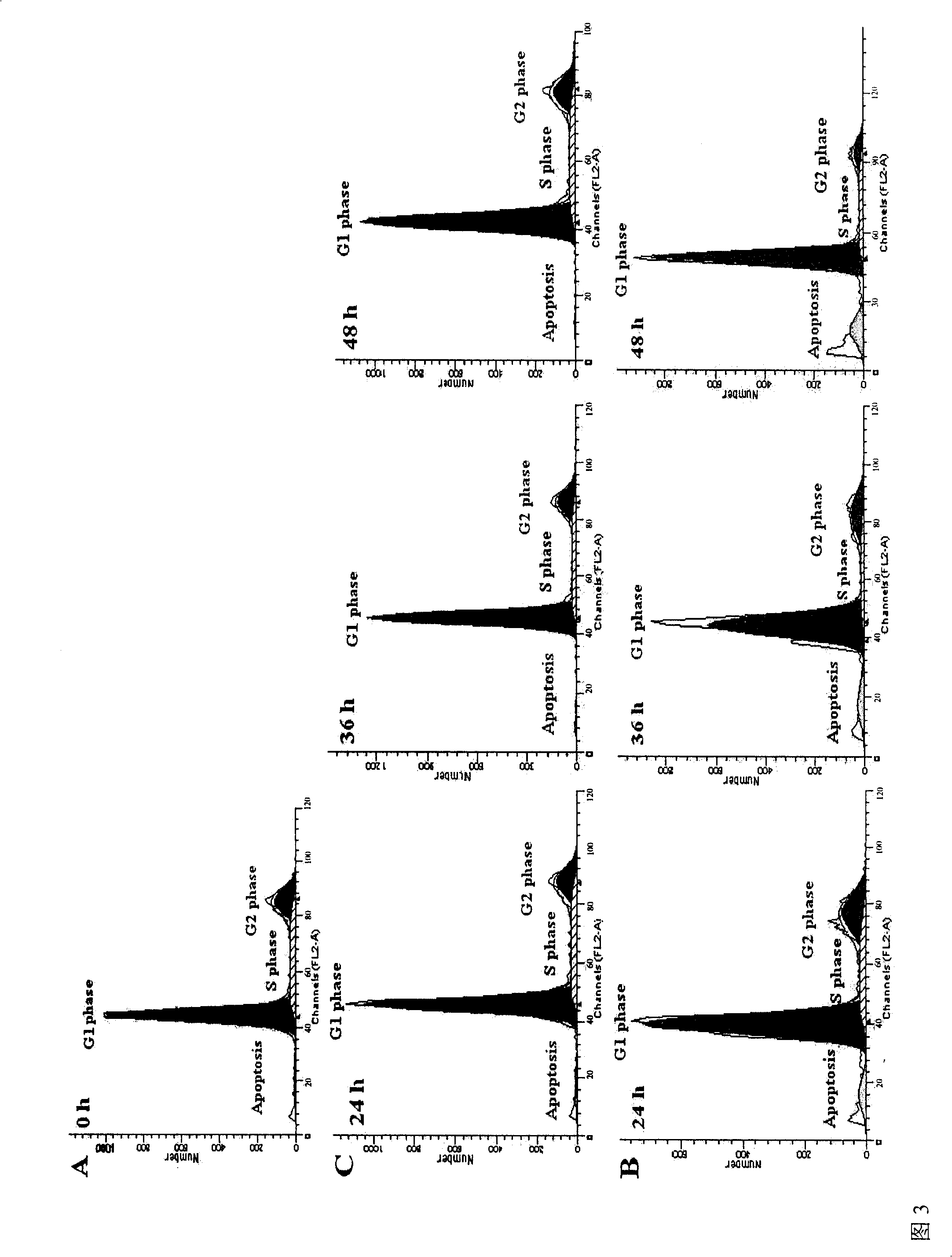
Application of Exenatide in preparing medicament for reversing activation of FFA to P53
A technology of activation and drugs, applied in the direction of drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as β-cell DNA damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Solid Phase Synthesis of Exenatide
[0022]Weigh 15.4g of Rink Amide MBHA resin and put it into the reaction bottle of the automatic peptide synthesizer, put each amino acid derivative, coupling reagent, and DMF solvent into each solvent bottle, set the reaction program and start the synthesis. The reaction temperature is 25°C, the coupling reaction time is 1 hour, the amino acid α-amino protecting group removal takes 30 minutes, the coupling and Fmoc group removal reaction solvent filling amount is 200ml, and the resin washing frequency is 3 minutes / time×5 Second-rate. When the coupling reaction of the last amino acid is completed, the resin is washed 5 times with methanol, and N 2 Let dry for 30 minutes. After each coupling and deprotection reaction, take out several resins to check the completion of the reaction. The detection reagent is A: the anhydrous ether solution of ninhydrin, the reagent B is the absolute ethanol solution of phenol, and the reagent C is the...
Embodiment 2
[0027] Preparation of free fatty acid FFA (palmitate-pal) compound BSA preparation:
[0028] The free fatty acid salt was mixed with 5% BSA phosphate buffer, and the mixture was added to the cell culture medium containing 10% calf serum, and the molar ratio of free fatty acid to BSA in the culture medium was 6.6:1. After adding the free fatty acid BSA mixture, the pH value of the medium did not change greatly, and the unbound free fatty acid remained in a soluble state during the experiment.
Embodiment 3
[0030] RINm-5F cell culture: Insulinoma cell RINm-5F (ATCC, CRL-11605 TM , USA) in RPMI1640 medium containing 10% fetal bovine serum, 3% (v / v) glutamine, 1 mM ketoglutarate sodium salt, 100 units / ml penicillin, 100 units / ml streptomycin and final glucose concentration Up to 16.8mM culture medium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com