Phosphonated fluoroquinolones, antibacterial analogs thereof, and methods for the prevention and treatment of bone and joint infections
A technology of fluoroquinolones and analogues, which is applied in the field of phosphonated fluoroquinolones, and can solve problems such as bone-specific drug delivery limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0365] Example 1: Synthesis of moxifloxacin, gatifloxacin and ciprofloxacin bisphosphonate conjugates
[0366] A) General experimental method
[0367] In Chem. Rev. (2005), 105:559-592, synthetic methods for the preparation of quinolone antibiotics are reviewed. The synthesis of moxifloxacin, gatifloxacin and ciprofloxacin are described in US Patent 4,990,517, US Patent 4,980,470 and US Patent 4,670,444, respectively.
[0368] A1) Preparation of bisphosphonate structural unit
[0369]
[0370] The general structure III can be obtained by alkylation of the anion of I with 4-substituted benzyl bromide II or bromoacetate IV following the protocol described in Bioorg. Med. Chem. (1999), 7:901-919 and the benzyl-substituted diphosphonate structural unit of V. By hydrogenation conditions, with a catalyst such as PtO 2 , the reduction of the nitro group converts the nitro compound IIIa to the aniline IIIb. Esters such as IIIc and Va can be converted to the corresponding aci...
Embodiment 2
[0959] Example 2 : In Vitro Antibacterial Activity and Cytotoxicity Assays
[0960] In vitro antibacterial activity
[0961] The susceptibility of Staphylococcus aureus (S. aureus) strains ATCC13709 and RN4220 to commercially available antibiotics and synthetic compounds was determined following guidelines established by the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) (M26-A). Serially diluted solutions of compounds were prepared in DMSO at two-fold dilutions and transferred into cation-adjusted Mueller Hinton broth (CAMHB; Becton Dickinson). Mix 50 μL of compound diluted with CAMHB with 100 μL of bacteria diluted with CAMHB in a 96-well microtiter plate. The final number of microorganisms in the assay is 5 x 10 5 c.f.u. / mL, the final concentration of DMSO in this assay was 1.25%. Assays were performed in duplicate and incubated at 37°C for 18h. The concentration of compound that inhibited visible bacterial ...
Embodiment 3
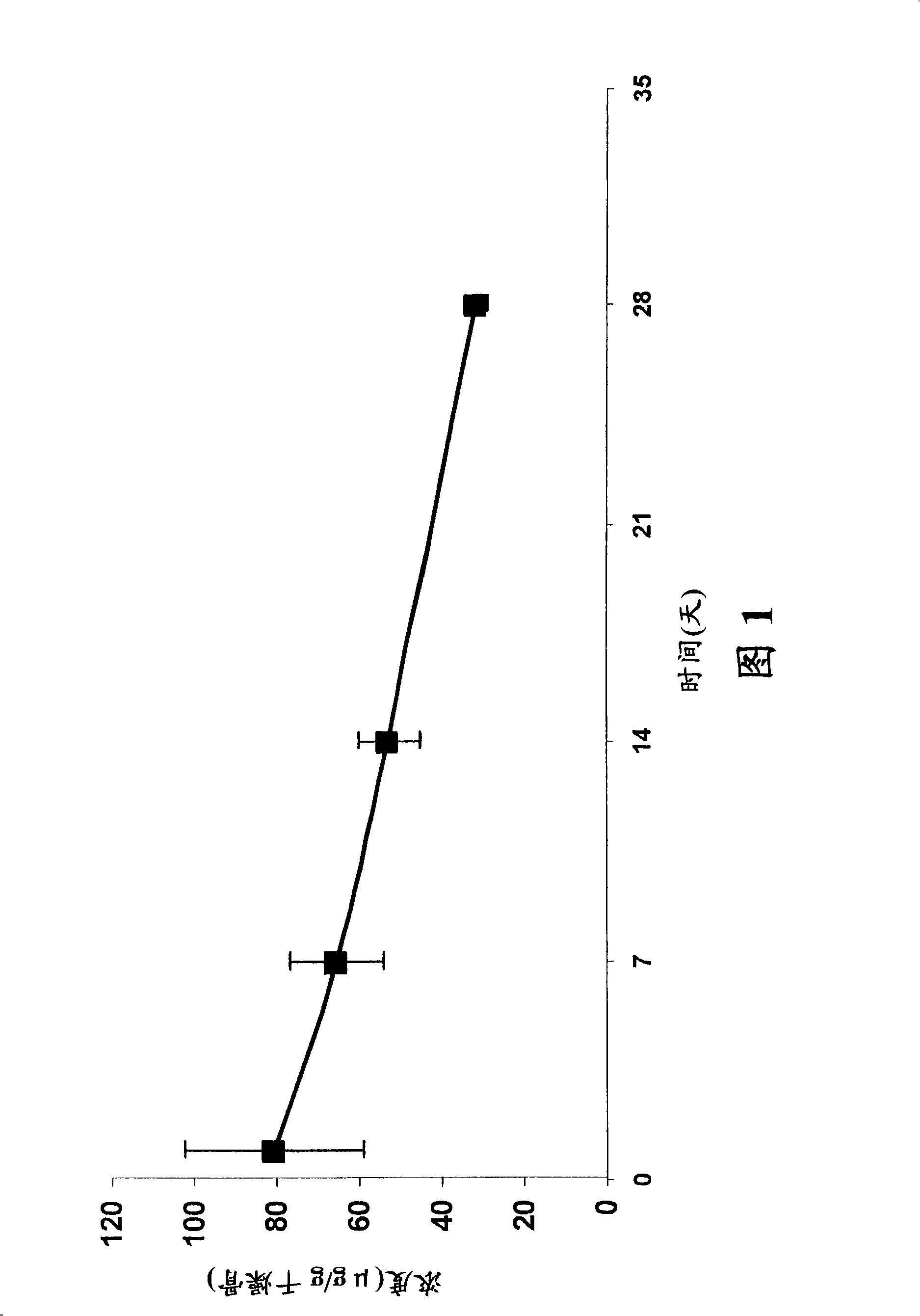
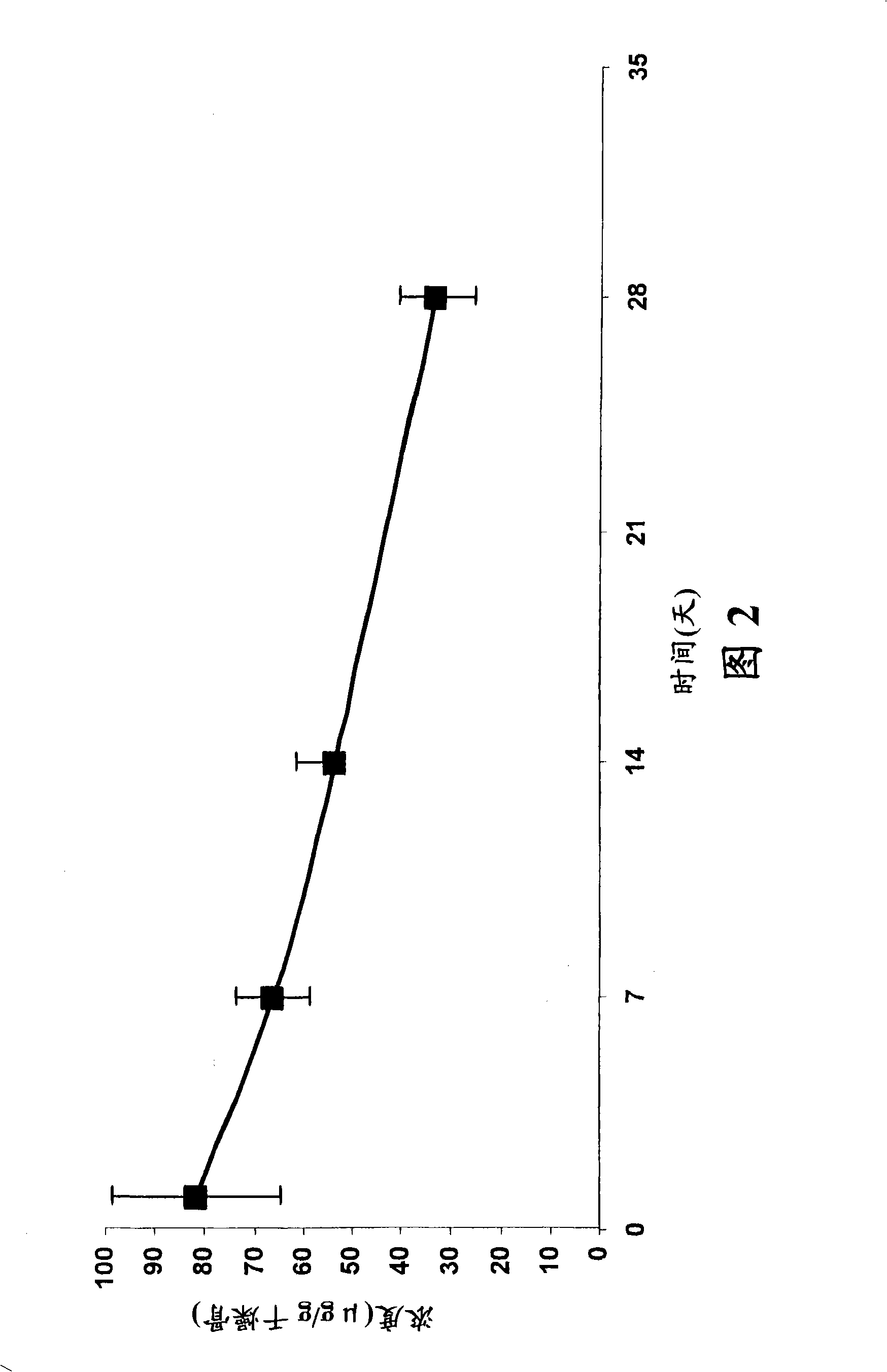
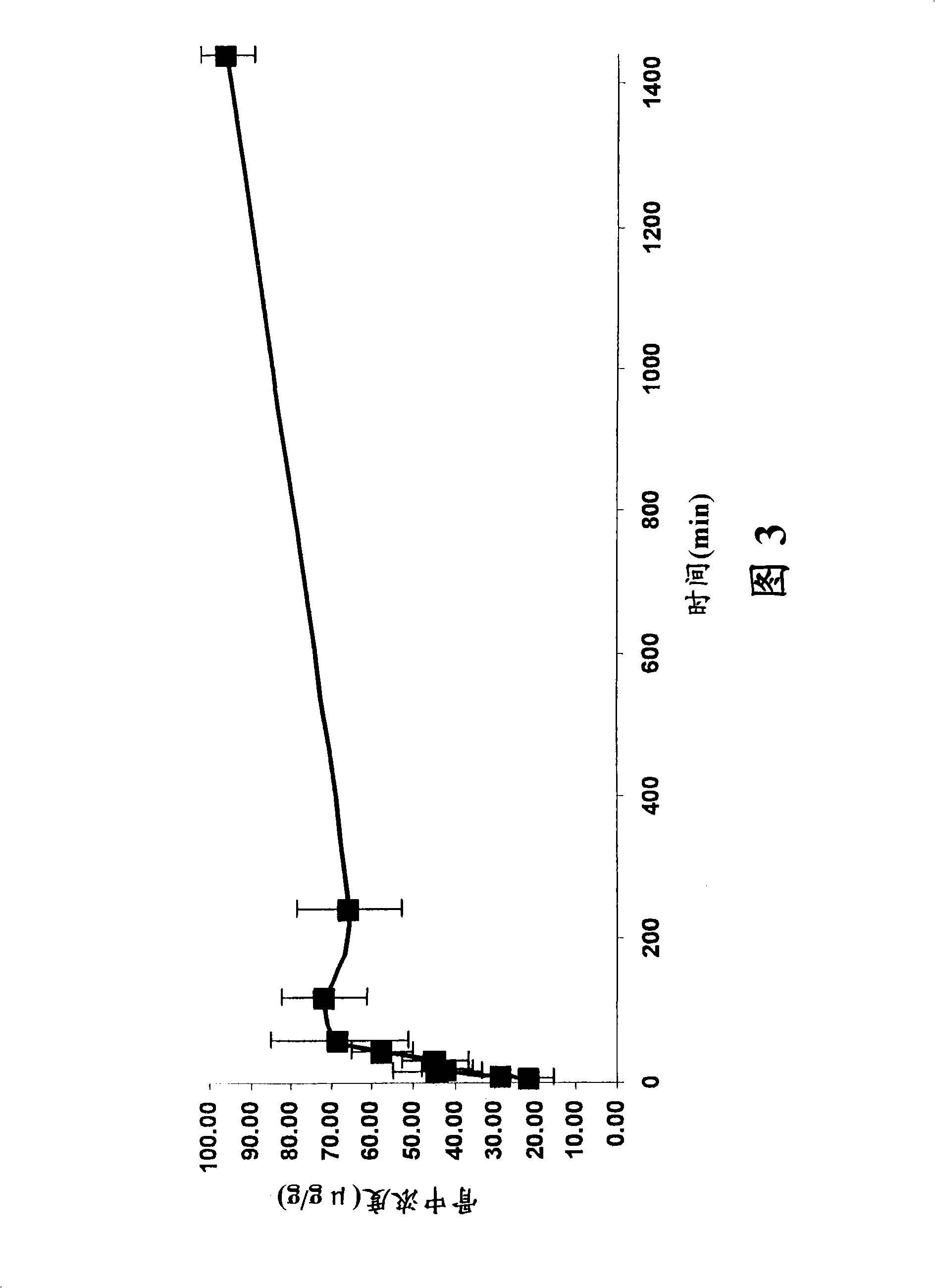
[0968] Example 3: Stability of fluoroquinolone-bisphosphonate conjugates
[0969] The stability of selected fluoroquinolone-bisphosphonate conjugates in solution and in different media was determined using LC / MS (liquid chromatography coupled to mass spectrometry) based methods or by bioassays. For LC / MS detection, 5 μL aliquots of 200 μM compound solutions were added to 95 μL medium (100 mM PBS (pH 7.5), 100 mM Tris (pH 7.5) or rat plasma serum). The mixture was incubated to various time points and then diluted with 500 μL of methanol. The mixture was shaken for 15 min and centrifuged at 10000 g for 15 min. Under a stream of argon, the supernatant was evaporated and the resulting residue was dissolved in 100 μL of water. The mixture was shaken for 15 min and centrifuged at 10000 g for 10 min. 20 [mu]L aliquots were then used to determine the concentration of parent drug by comparison to LC / MS standards. The LC / MS analysis method is based on Zorbax TM Agilent 1100 with S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com