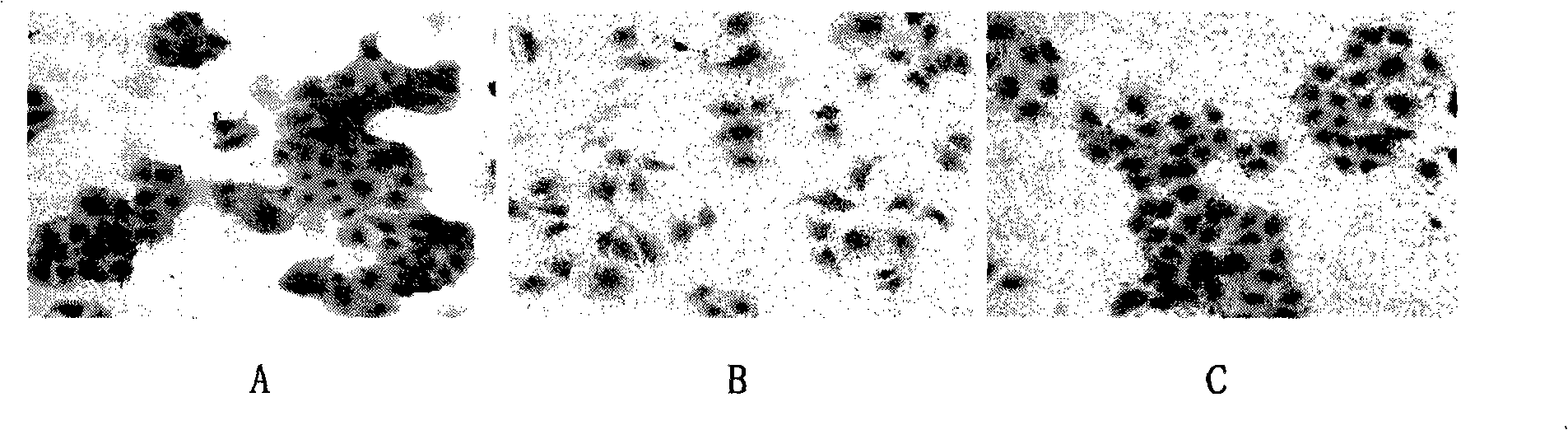
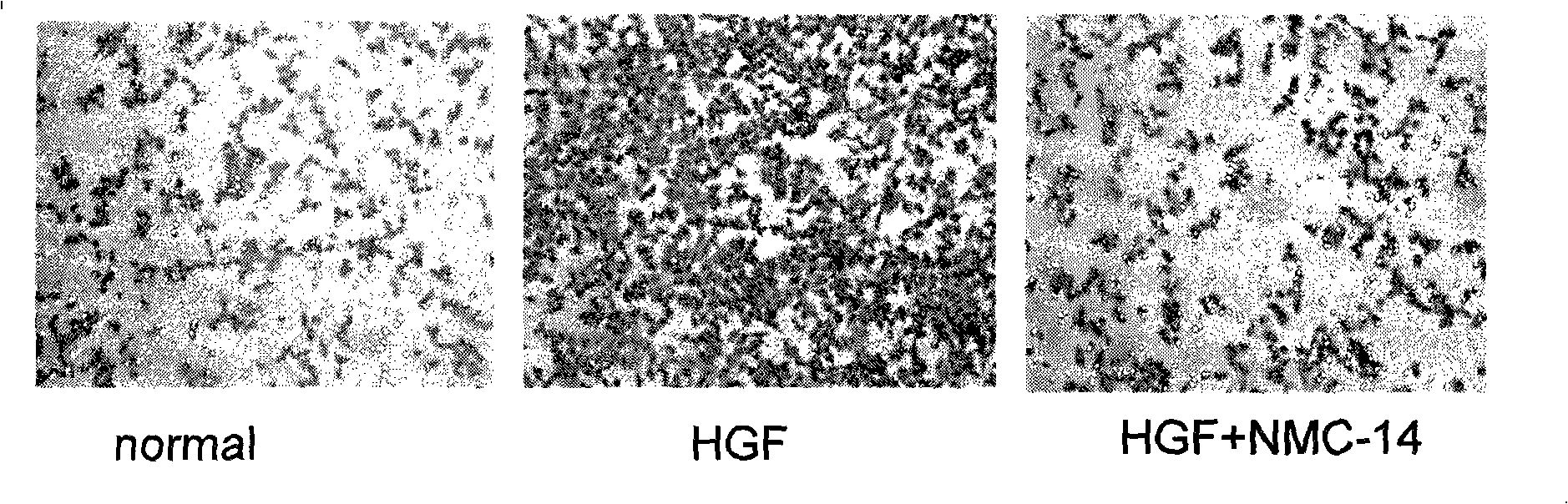
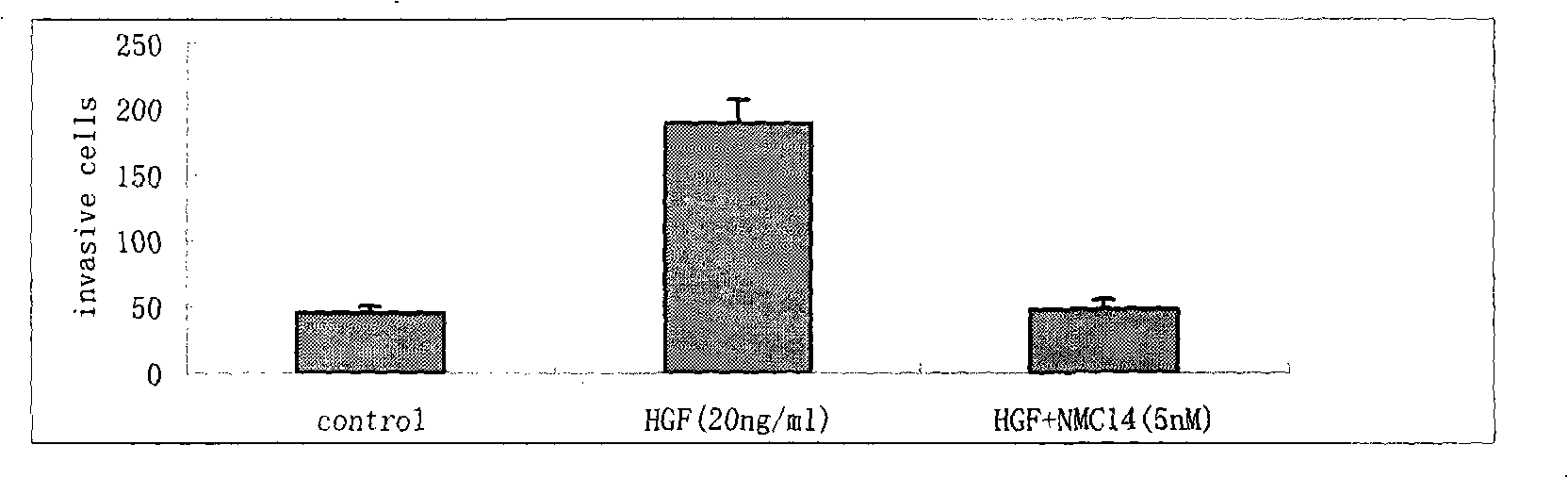
HGF/c-Met signalling channel restrainer, preparation method and application thereof
A general formula and solvate technology, applied in the field of prevention and treatment of tumor diseases, can solve the problems of high toxicity and side effects, poor bioavailability, poor selectivity, etc., and achieve the effect of inhibiting invasion and growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0294] The preparation of M24 adopts the classic Knorr reaction, and the corresponding oxime is obtained by reacting tert-butyl acetoacetate with an equivalent amount of sodium nitrite in acetic acid at 10°C for 2 hours. Stand at room temperature to obtain granular M23 solid. Then heat the acetic acid solution of ethyl acetoacetate to 60°C, start the mechanical stirring, and add the acetic acid solution of zinc powder and oxime in turn from the left and right ports of the three-necked bottle respectively, and do not let the temperature exceed 75°C during the addition (otherwise, the temperature will rapidly Rising, if not controlled in time, bumping will occur rapidly and rush out of the reaction bottle, which is very dangerous), after the addition is completed, react at 75-80°C for 1 hour, and after cooling to room temperature, pour the reaction solution into 10 times the volume of ice-water mixture , Stirring while adding, the waxy solid was precipitated rapidly. After filt...
Embodiment 1
[0322] Example 1 3-(2'hydroxyphenylmethylene)-5-(α-phenethylaminosulfo)-2-indolone;
[0323] In a 10ml eggplant-shaped bottle, add 80mg (0.65mmol) of salicylaldehyde and 5-(α-phenethylaminosulfo)-2-indolinone; 161mg (0.50mmol), add 6ml of absolute ethanol, piperidine 2 drops, then the temperature was raised to reflux, and the reaction was stopped after stirring and reacting for 20 minutes under reflux. After standing at room temperature for 2 hours, flash chromatography gave 101 mg of the target product (eluent petroleum ether: ethyl acetate = 1:1), with a yield of 51%. 1 H-NMR (DMSO-d 6 )δ(ppm): 10.95(s, 1H), (s, 1H), 8.09(m, 1H), 7.78(m, 2H), 6.88-7.52(m, 11H), 4.27(q, 1H, J= 6.4 Hz), 1.18 (d, 3H, J = 6.4 Hz). FAB-Ms: 421.0 (M + +1).
Embodiment 2
[0324] Example 2 3-(5'-methoxy-indole-3'-methylene)-5-(α-phenethylaminosulfo)-2-indolone;
[0325] In a 10ml eggplant-shaped bottle, add 80 mg (0.50 mmol) of 5-methoxy-3-indole aldehyde and 5-(α-phenethylaminosulfo)-2-indolinone; 161 mg (0.50 mmol), add 6ml of absolute ethanol, 2 drops of piperidine, and then the temperature was raised to reflux, and the reaction was stirred and reacted under reflux for 20 minutes, and then the reaction was stopped. After standing at room temperature for 5 hours, 121 mg of the target product was precipitated, with a yield of 54%. FAB-Ms: 473.0 (M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com