Method for preparing magnesium sulfuric acid monohydrate with mixture salt of sodium chloride and epsomite
A sodium chloride and mixed salt technology, applied in directions such as magnesium sulfate, can solve the problems of high energy consumption, high production cost, heavy environmental pollution, etc., and achieve the effects of low energy consumption, low production cost, and no environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
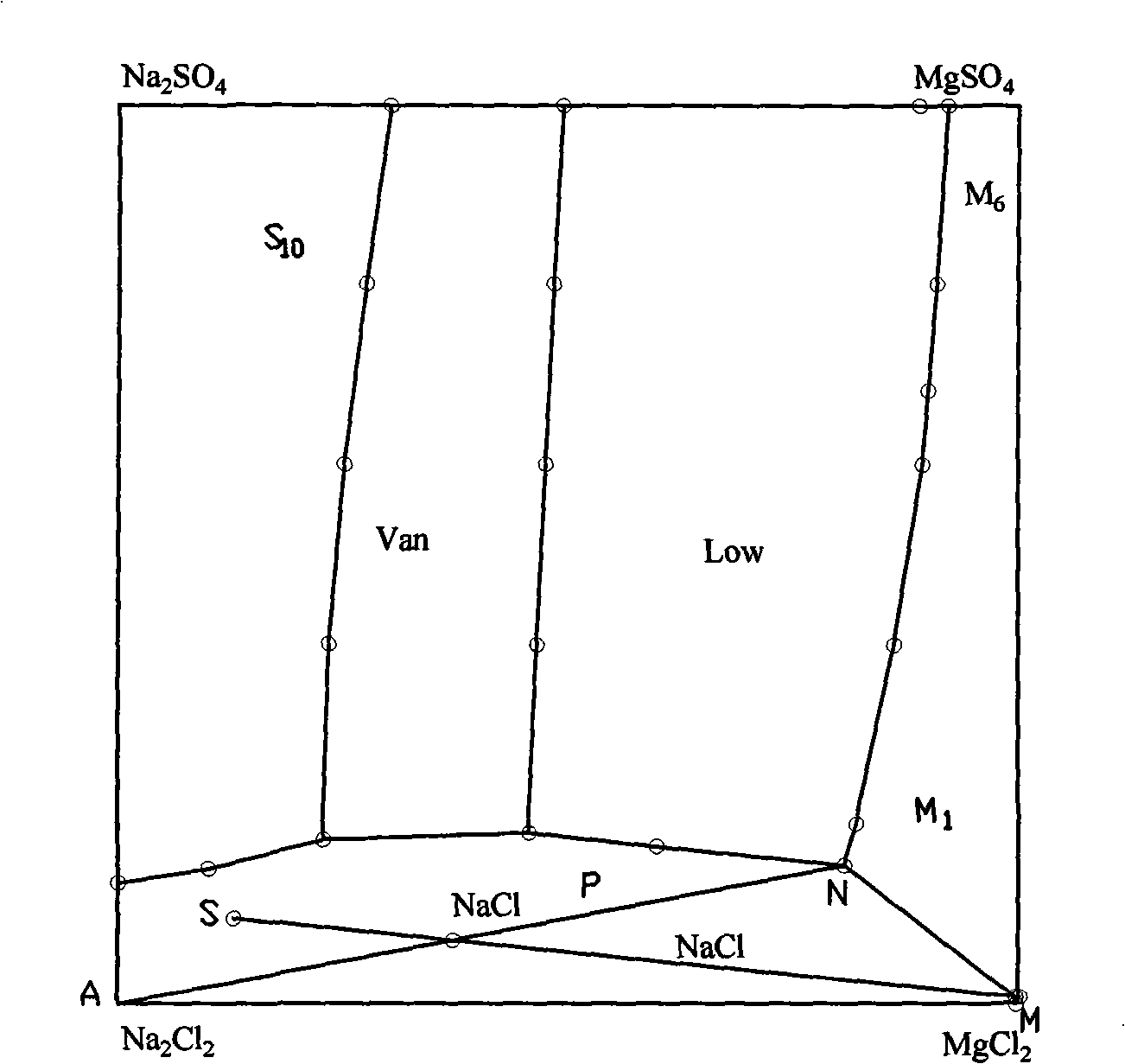
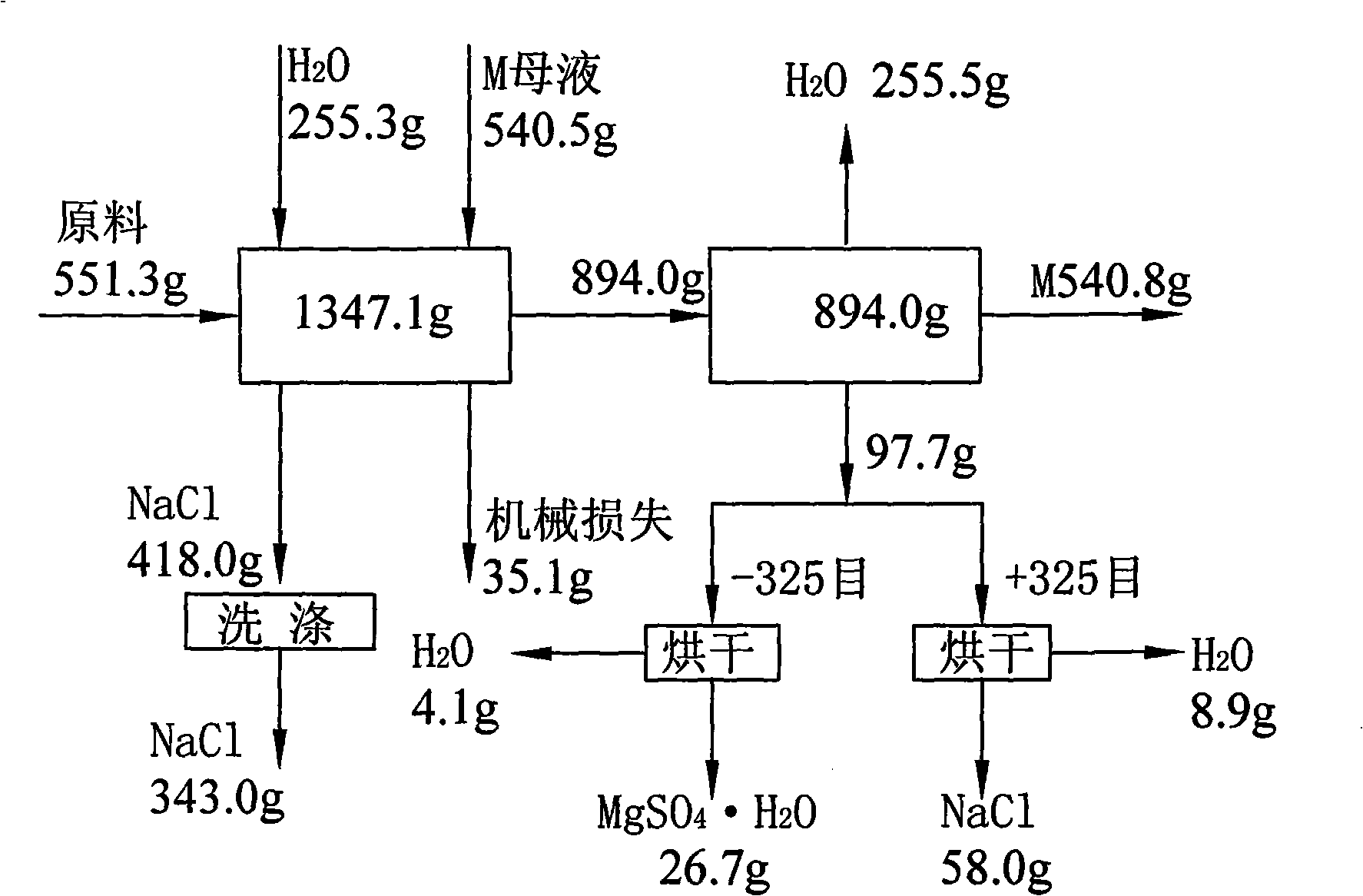
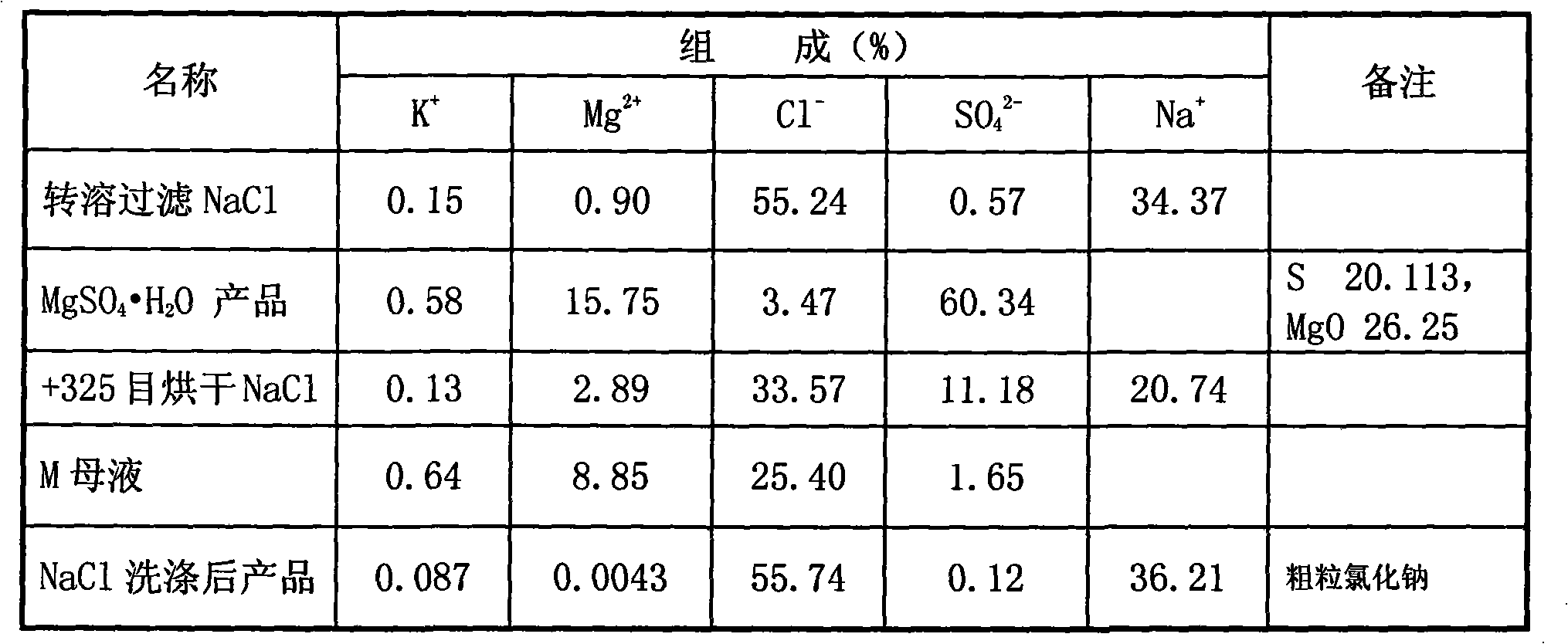
[0016] (1) fully mix the mixed salt of raw material sodium chloride and epsom salt (for extracting the tailings after potassium and magnesium fertilizer), the specific chemical composition of raw material is shown in Table 1; (2) batching, take mixed salt 551.32g, Add 255.34g of fresh water, 540.46g of bittern (the discharge mother liquor after evaporating and crystallizing potassium chloride and langbeinite in the processing of salt fields, commonly known as old brine, and the main component is magnesium chloride), and stir evenly; (3) heat to 75° C. (4) filter out the undissolved NaCl; (5) heat the mother liquor after filtering off the undissolved NaCl to 75°C, and concentrate by vacuum multi-effect evaporation until about 338.2g of water is evaporated ( Keep the temperature at 75°C, and the evaporation time is about 30 minutes), crystallize fine-grained magnesium sulfate monohydrate and coarse sodium chloride from the mother liquor; (7) filter to obtain fine-grained magnesiu...
Embodiment 2
[0023] (1) Fully mix the mixed salt of raw material sodium chloride and Epsom salt (tailings after extracting potassium and magnesium fertilizer), the specific chemical composition of the raw material is shown in Table 3; (2) ingredients, weigh 546.12g of mixed salt, add 247.05g of fresh water, 536.01g of brine (the mother liquor discharged from salt fields after processing and evaporating crystallized potassium chloride and lenbeinite, commonly known as old brine, the main component is magnesium chloride), and stir evenly; (4) filter out the undissolved NaCl; (5) heat the mother liquor after filtering off the undissolved NaCl to 75° C., and concentrate by vacuum multi-effect evaporation until about 334.1 g of water (temperature Keep it constant at 75°C, and the evaporation time is 30 minutes), crystallize fine-grained magnesium sulfate monohydrate and coarse-grained sodium chloride from the mother liquor; (7) filter to obtain a mixture of fine-grained magnesium sulfate monohyd...
Embodiment 3
[0030] (1) Fully mix the mixed salt (tailings after extracting potassium and magnesium fertilizer) of raw material sodium chloride and epsom salt, the specific chemical composition of the raw material is shown in Table 5; (2) ingredients, weigh 554g of mixed salt, add fresh water 256g, 544.5g of brine (the discharge mother liquor after evaporating crystalline potassium chloride and magnesite in the salt fields, commonly known as old brine, the main component is magnesium chloride), stir evenly; (3) heat to 75°C, heat-dissolve for half an hour, Promote the dissolution of mixed salts; (4) filter out the undissolved NaCl; (5) heat the mother liquor after filtering out the undissolved NaCl to 75°C, and concentrate by vacuum multi-effect evaporation until about 335.2g of water is evaporated (the temperature is maintained at 75 ℃ constant, about 30 minutes of evaporation time), from the mother liquor crystallization fine magnesium sulfate monohydrate and coarse sodium chloride; (7) f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com