Novel synthesis process for dimethyl fumarate
A technology of dimethyl fumarate and a new process, which is applied in the direction of carboxylic acid ester preparation, organic compound preparation, organic compound/hydride/coordination complex catalyst, etc., and can solve the problems of increasing equipment investment, equipment corrosion, reaction Long time and other problems, to achieve the effect of saving equipment investment, reducing raw material costs, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
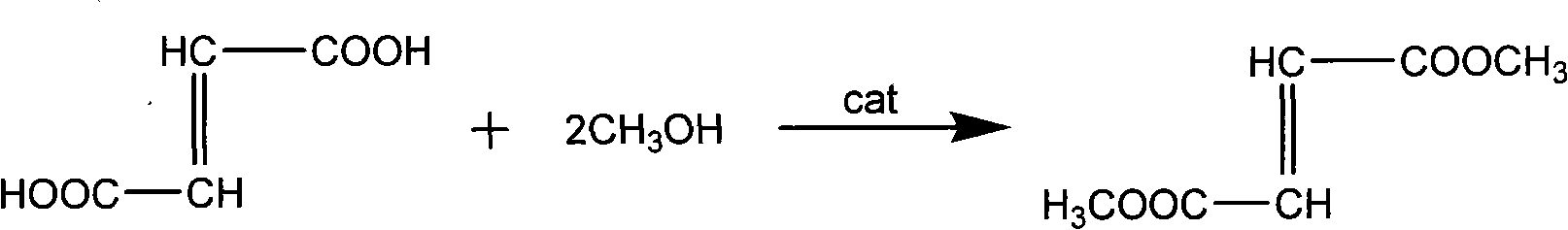
[0021] Add 116.0g (1.0mol) fumaric acid, 96.12g (3.0mol) methanol, 1.24g (0.0025mol) cerous methanesulfonate in sequence to a 500mL three-necked reaction flask equipped with a reflux condenser, and heat to reflux for about 3 hours Afterwards, remove the water that methyl alcohol and reaction generate, then add 96.12g (3.0mol) methyl alcohol in the reaction flask and continue heating and reflux reaction 4h, filter while hot and reclaim cerous methanesulfonate (the cerous methanesulfonate that reclaims can reuse). The filtrate was washed with water, allowed to stand, cooled, crystallized, filtered with suction and dried to obtain dimethyl fumarate in the form of white crystals. Product quality 137.95g (yield: 95.8%), melting point 102~104 ℃.
[0022] The reaction equation is:
[0023]
[0024] Wherein, above-mentioned cerous methanesulfonate can be synthesized by following method:
[0025] Weigh 14.4g of methanesulfonic acid, mix it with water at a volume ratio of 1:1 and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com