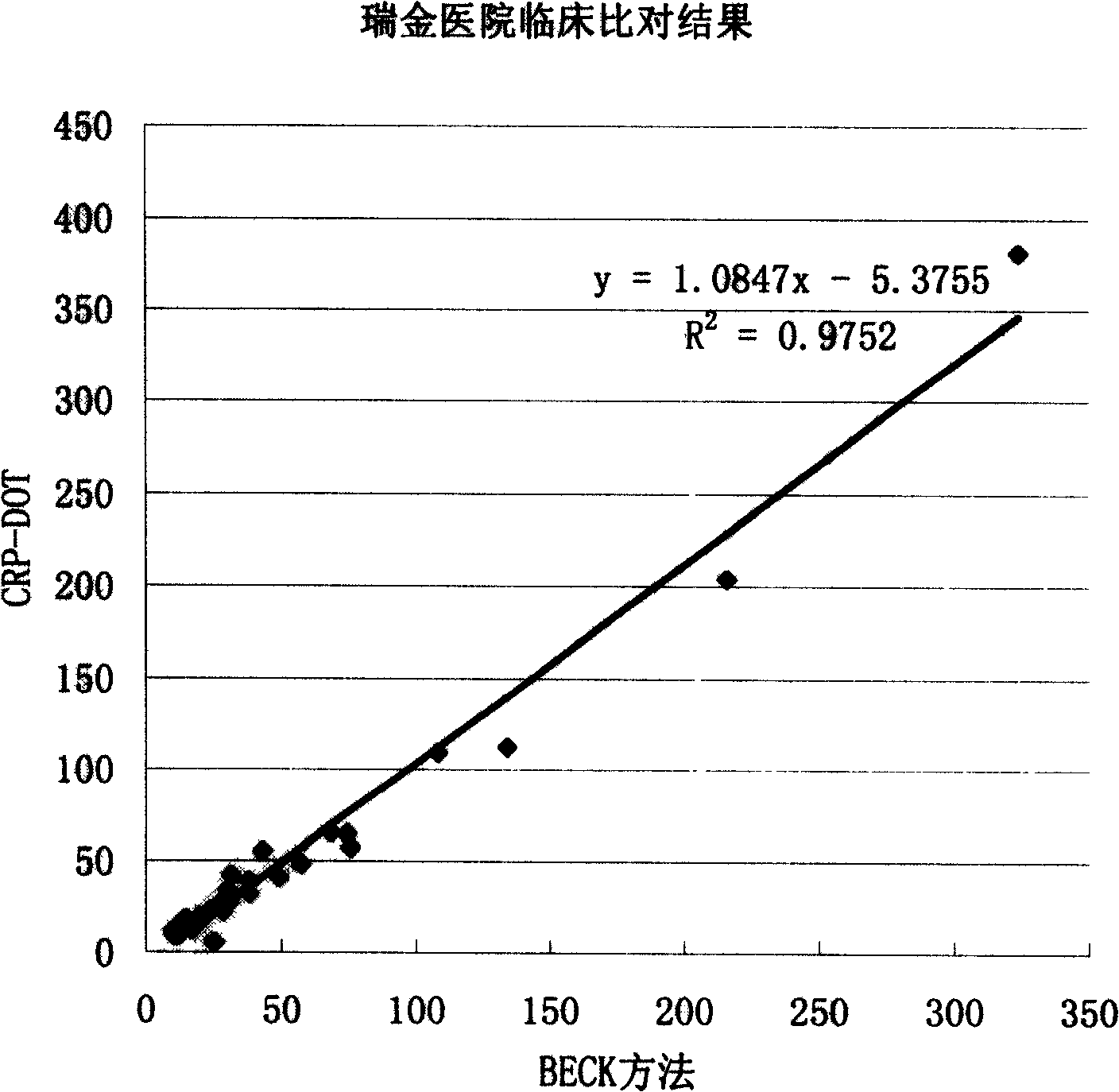
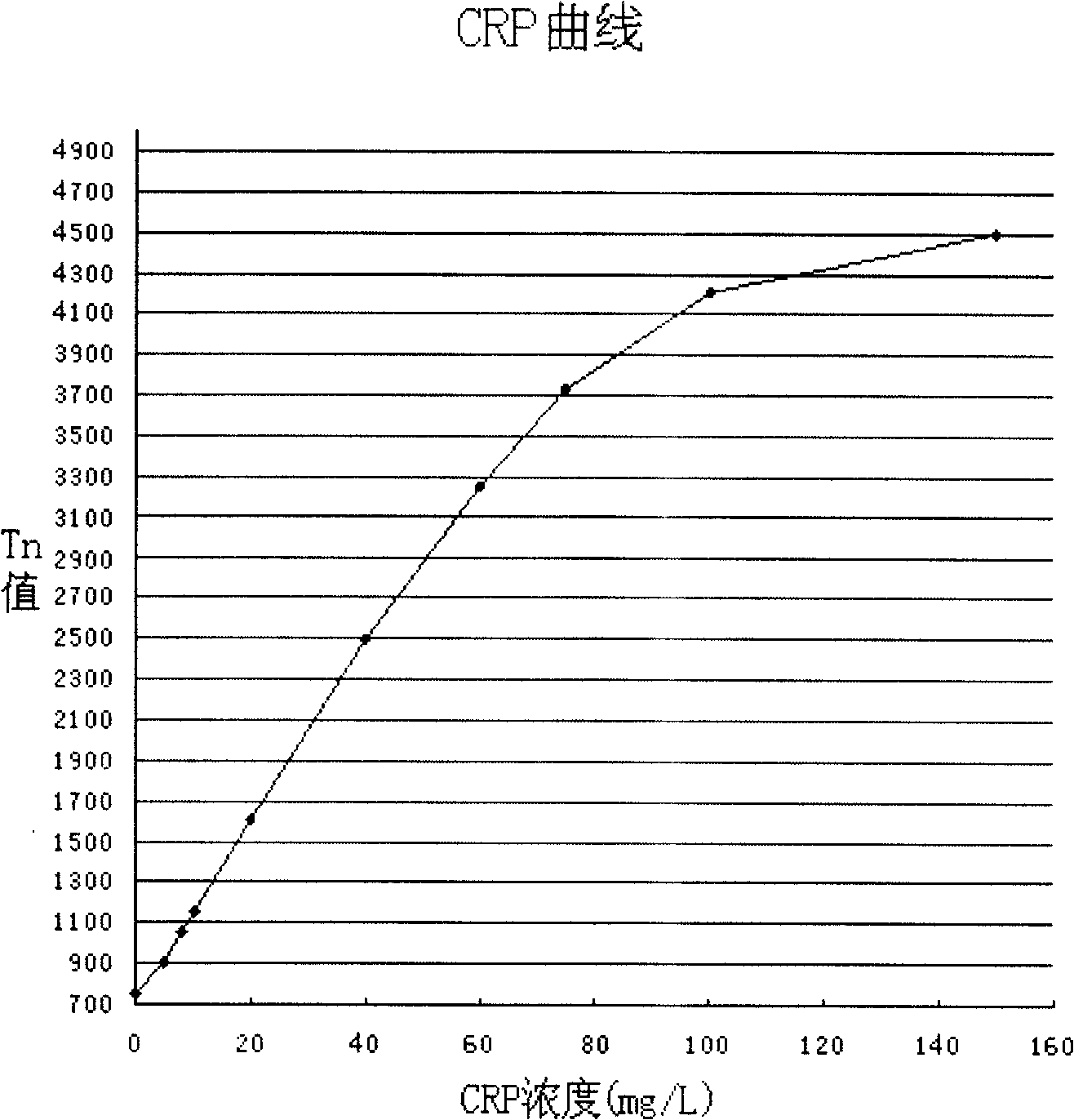
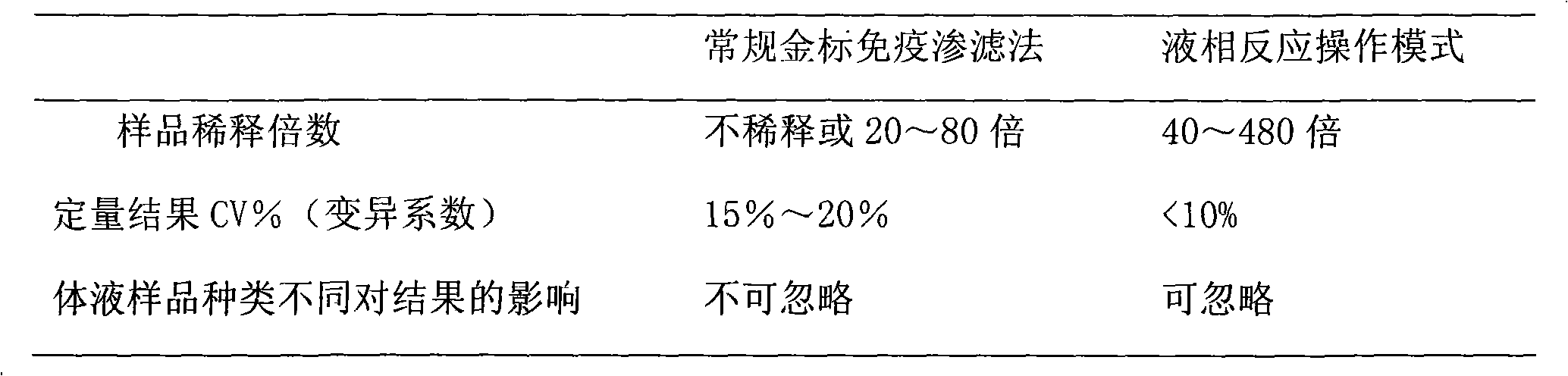
Colloidal gold method for fast quantitative determination of C-reaction protein and its application
A quantitative detection and reactive protein technology, applied in the field of clinical immunology detection, can solve the problems of long detection time, poor reaction homogeneity, unsuitable for single test, etc., to shorten the cure time and reduce medical expenses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Example 1 uses the preparation of the CRP gold standard method rapid quantitative detection reagent of two monoclonal antibodies
[0032]1. Preparation of freeze-dried anti-CRP immune colloidal gold and reaction plate: according to the method introduced by Lu Shengkai et al. (Chinese Journal of Microbiology and Immunology, 13(2):125, 1993; Shanghai Journal of Medical Laboratory, 5(1):62 , 1990) prepared colloidal gold and anti-CRP immune colloidal gold conjugates. However, the colloidal gold is coated with purified CRP monoclonal antibody, 0.15mg of CRP monoclonal antibody is used to coat 10ml of colloidal gold, and 2500ml of colloidal gold is finally recovered to 1500ml anti-CRP immune colloidal gold application solution, buffer containing 1% BSA, 0.5mg / ml PEG 20M, 0.1% sodium azide. The pre-cooling temperature for freeze-drying is -35°C, the maximum temperature rise is 30°C, and the vacuuming time is 20 hours. The filling volume of the bottle can be set as required, ...
example 2
[0041] Example 2 CRP colloidal gold method rapid quantitative detection method
[0042] 1. Take out the reagent from the cold storage place, equilibrate at room temperature for at least half an hour, and accurately add 1.1ml of CRP freeze-dried product reconstituted solution to the anti-CRP gold standard solution freeze-dried product and shake well.
[0043] 2. Use a clean tip to absorb 100 μl of the reconstituted CRP gold standard solution and add it to an empty reaction tube. The tip only absorbs the CRP gold standard solution to prevent any contamination.
[0044] 3. Place the CRP reaction plate flat on the test bench, add 2 drops of CRP blocking solution to the reaction well, and wait until it is completely infiltrated
[0045] 4. Fresh serum samples do not need to be pretreated. Serum that has been frozen or stored at 4-8°C for several days and centrifuged can be used as the supernatant; accurately pipette 10 μl of serum and add it to the CRP dilution tube (see the bottle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com