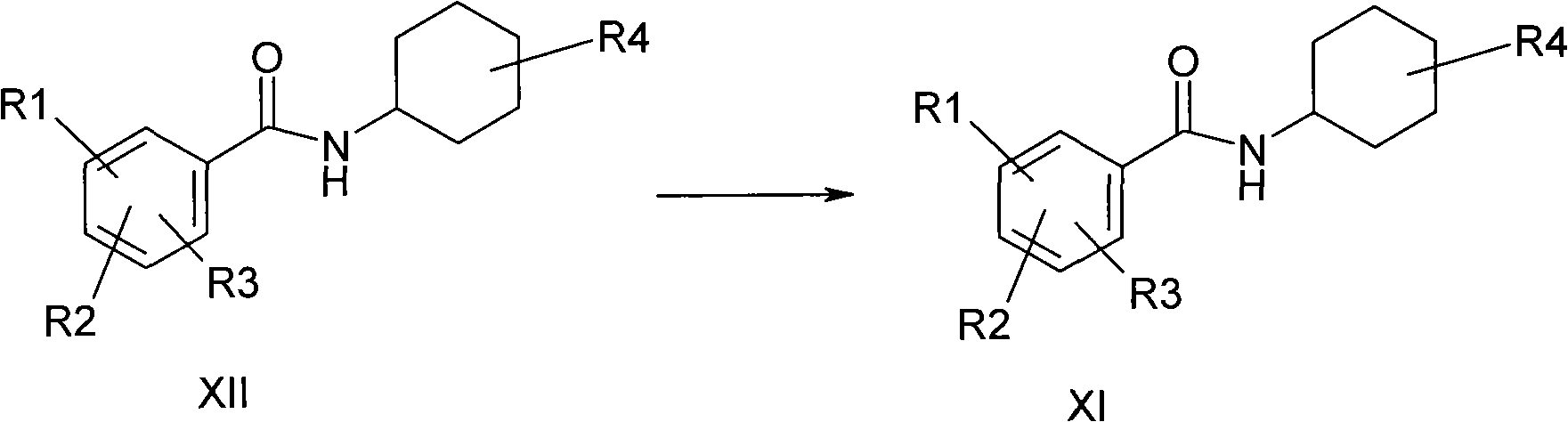
Process for preparing ambroxol, analogue thereof or salts thereof
A synthesis method and amino technology are applied in the synthesis field for preparing ambroxol hydrochloride, and can solve the problems of reduced yield, complicated operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
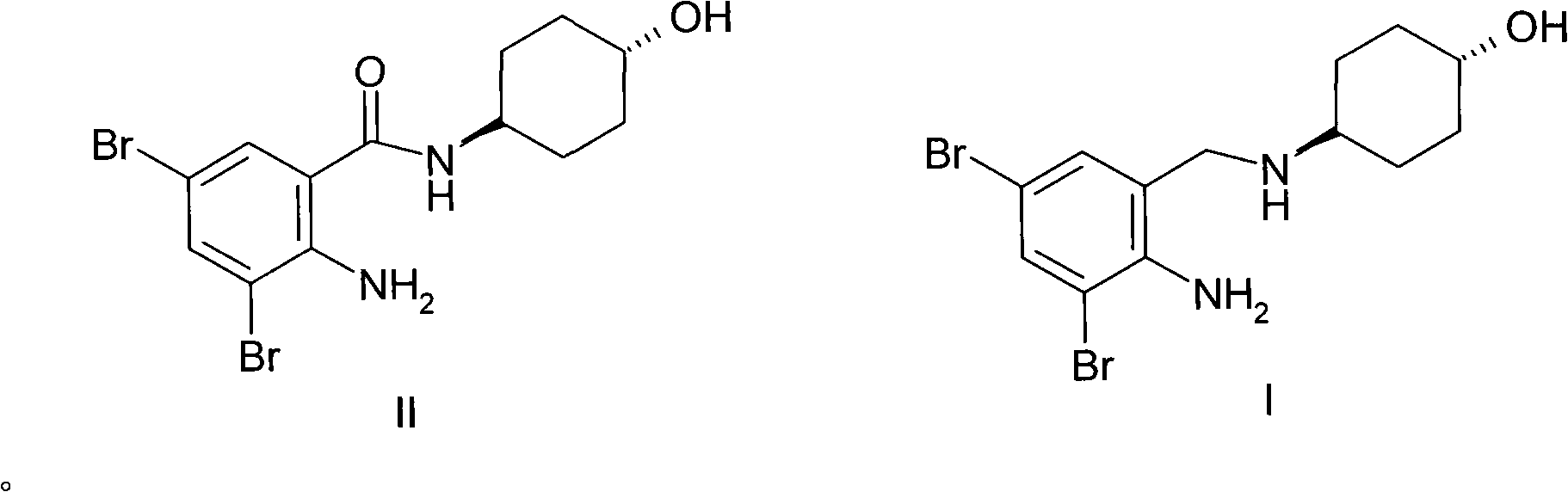
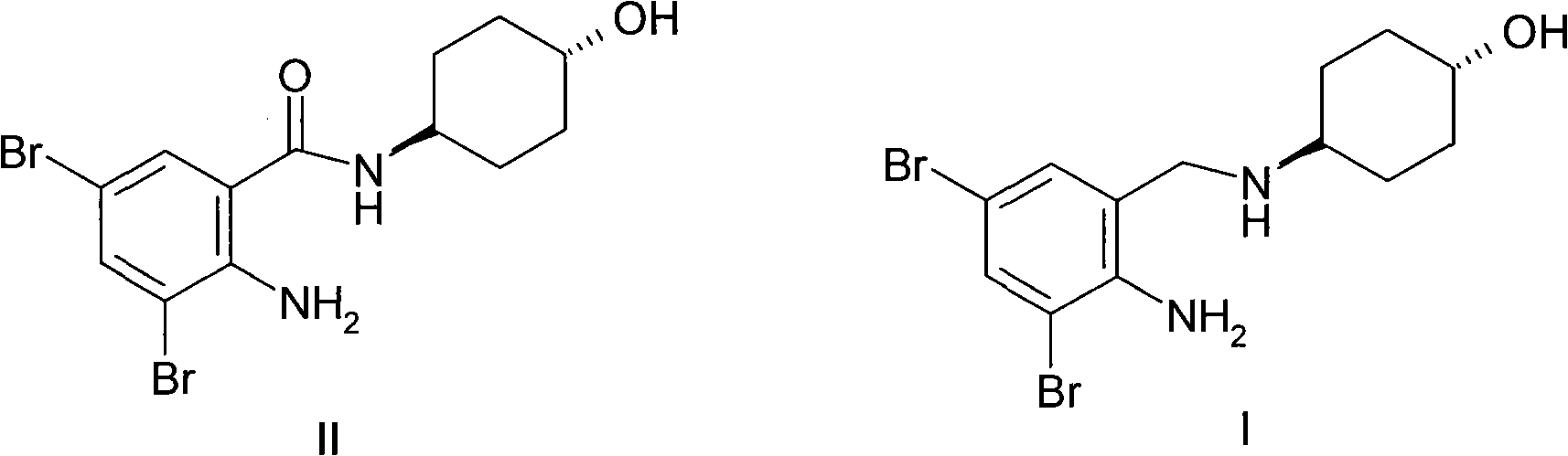
[0019] Preparation Example 1 N-(trans-4-hydroxycyclohexyl)-2-amino-3,5-dibromobenzamide (II)
[0020]
[0021] According to the method reported in the literature (ES 8605219), N-(trans-4-hydroxycyclohexyl)-2-amino-3,5 -dibromobenzamide (II), its three-step total yield is 61%.
Embodiment 1
[0022] Example 1 N-(trans-4-hydroxycyclohexyl)-N-(2-amino-3,5-dibromobenzyl)amine (I) (LiCl method)
[0023]
[0024] Dissolve N-(trans-4-hydroxycyclohexyl)-2-amino-3,5-dibromobenzamide (II, 3.90g, 0.010mol) in 120ml of dry tetrahydrofuran, and cool to 0 ℃, add 0.46g (0.011mol) of anhydrous lithium chloride to form a suspension, add 0.25g (0.007mol) of sodium borohydride, keep 0℃ for 4 hours, rise to room temperature, evaporate the solvent under reduced pressure, and use the residue Extract with 80ml of ethanol, acidify the extraction solution with concentrated hydrochloric acid, add a small amount of ether, and crystallize the crude product of ambroxol hydrochloride. The crude product was recrystallized from ethanol-ether to obtain 3.65 g of pure ambroxol hydrochloride (88% yield). Mp: 216-217.5. ESI MS: 379 (M+1).
Embodiment 2
[0025] Example 2 N-(trans-4-hydroxycyclohexyl)-N-(2-amino-3,5-dibromobenzyl)amine (I)(TiCl 4 Law)
[0026] Dissolve N-(trans-4-hydroxycyclohexyl)-2-amino-3,5-dibromobenzamide (II, 3.97g, 0.012mol) in 120ml of dry tetrahydrofuran, and cool to 0 ℃, add 1.40ml (0.013mol) of titanium tetrachloride, add 0.30g (0.008mol) of sodium borohydride, keep the reaction at 0℃ for 4 hours, rise to room temperature, evaporate the solvent under reduced pressure, and extract 80ml of the residue with ethanol to extract The solution was acidified with concentrated hydrochloric acid, and a small amount of ether was added to crystallize crude ambroxol hydrochloride. The crude product was recrystallized from ethanol-ether to obtain 4.75 g of pure ambroxol hydrochloride (95% yield). MP: 216-217. ESI MS: 379 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com