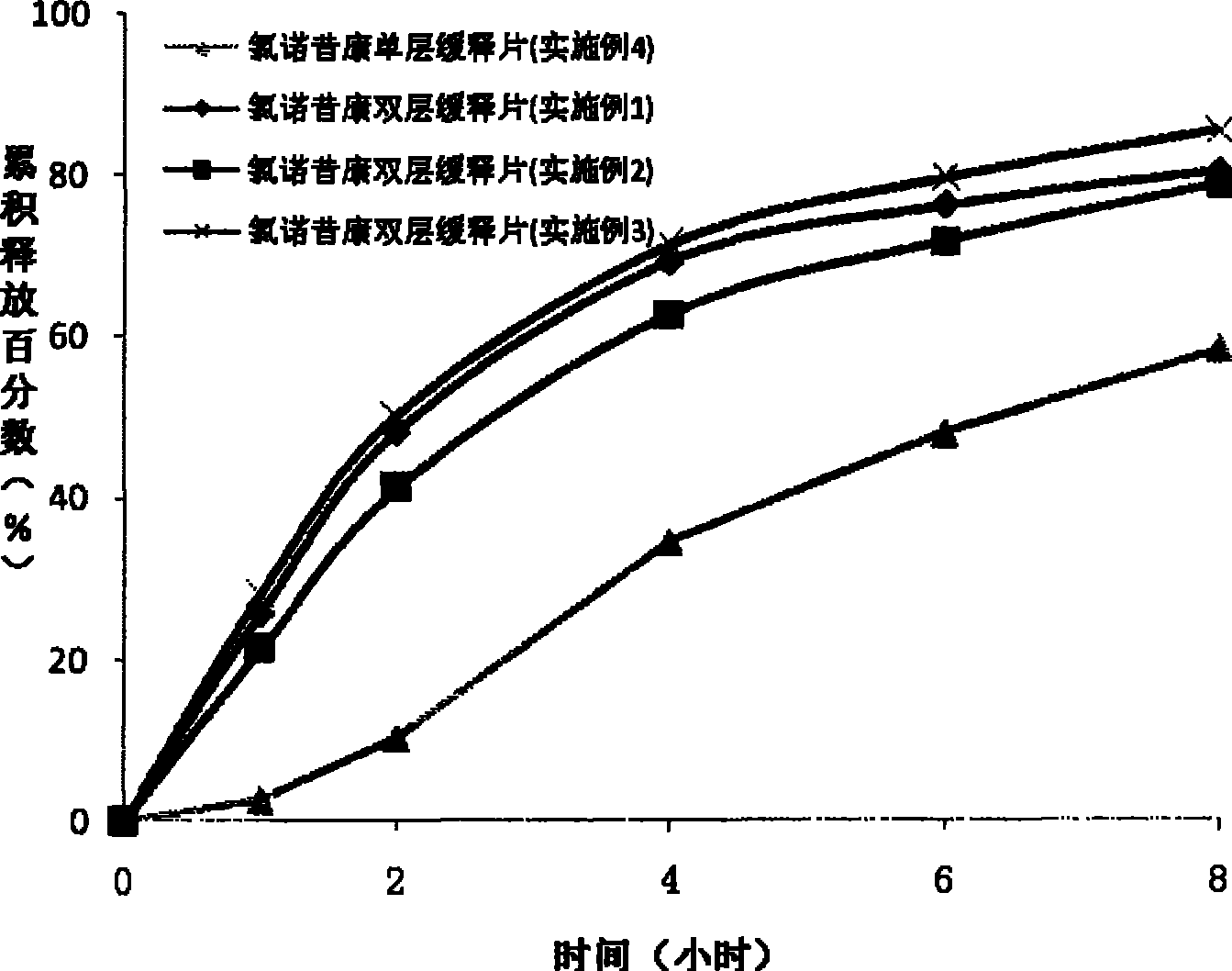
Lornoxicam double-layer sustained release tablets
A technology of lornoxicam and sustained-release tablets, which is used in pharmaceutical formulations, pill delivery, non-central analgesics, etc. Problems such as low concentration and difficult treatment concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0145] The preparation method comprises the following steps:
[0146] (i) providing an immediate release layer material and a sustained release layer material, wherein
[0147] The immediate-release layer material comprises: (a1) lornoxicam; (a2) a basic substance; (a3) optional other pharmaceutically acceptable carriers or excipients;
[0148] The sustained-release layer material comprises: (b1) lornoxicam; (b2) sustained-release material; (b3) optional other pharmaceutically acceptable carriers or excipients; and
[0149] (ii) compressing the immediate-release layer material and the sustained-release layer material into a double-layer sustained-release tablet.
[0150] The content and ratio of each material component are as defined above.
[0151] For example, in a preferred embodiment of the present invention, a wet granulation and tableting method is adopted, and its specific technological process is:
[0152] A. Pretreatment of raw and auxiliary materials
[0153] P...
Embodiment 1
[0175] Example 1 Lornoxicam bilayer sustained-release tablet 1
[0176] formula
[0177] Immediate release layer part (amount per tablet)
[0178] lornoxicam 4 mg
[0179] L-Arginine 16 mg
[0180] Croscarmellose Sodium 10mg
[0181] Pregelatinized starch 84 mg
[0182] 5% Povidone 30mg
[0183] Magnesium Stearate 1 mg
[0184] Sustained release layer part (amount per tablet)
[0185] lornoxicam 8 mg
[0186] Hypromellose (viscosity: 4000 cps) 15 mg
[0187] Hypromellose (viscosity: 100 cps) 30 mg
[0188] Lactose 122 mg
[0189] 5% Povidone (Model: K30) solution 80 mg
[0190] Magnesium Stearate 1.8 mg
[0191] Preparation
[0192] (1) Preparation of immediate-release granules:
[0193] Pass lornoxicam, pregelatinized starch, L-arginine and croscarmellose sodium through a 60-mesh sieve and mix well. Add 5% povidone (g / 100ml, model: K30) solution during stirring to make soft material, pass through a 30-mesh sieve for granulation, dry at 50°C for 1 hour, pass...
Embodiment 2
[0198] Example 2 Lornoxicam bilayer sustained-release tablet 2
[0199] formula
[0200] Immediate release layer part (amount per tablet)
[0201] lornoxicam 6mg
[0202] L-Arginine 22 mg
[0203] Croscarmellose Sodium 15mg
[0204] Pregelatinized starch 105 mg
[0205] 5% Povidone 40mg
[0206] Magnesium Stearate 1.5 mg
[0207] Sustained release layer part (amount per tablet)
[0208] lornoxicam 12mg
[0209] Hypromellose (viscosity: 4000 cps) 20 mg
[0210] Hypromellose (viscosity: 100 cps) 50 mg
[0211] Lactose 105 mg
[0212] 5% Povidone (Model: K30) solution 80 mg
[0213] Magnesium Stearate 2.3 mg
[0214] Preparation
[0215] (1) Preparation of immediate-release granules:
[0216] Pass lornoxicam, pregelatinized starch, L-arginine and croscarmellose sodium through a 60-mesh sieve and mix well. Add 5% povidone ((g / 100ml, model: K30) solution during stirring to make soft material, pass through a 30-mesh sieve for granulation, dry at 50°C for 1 hour, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com