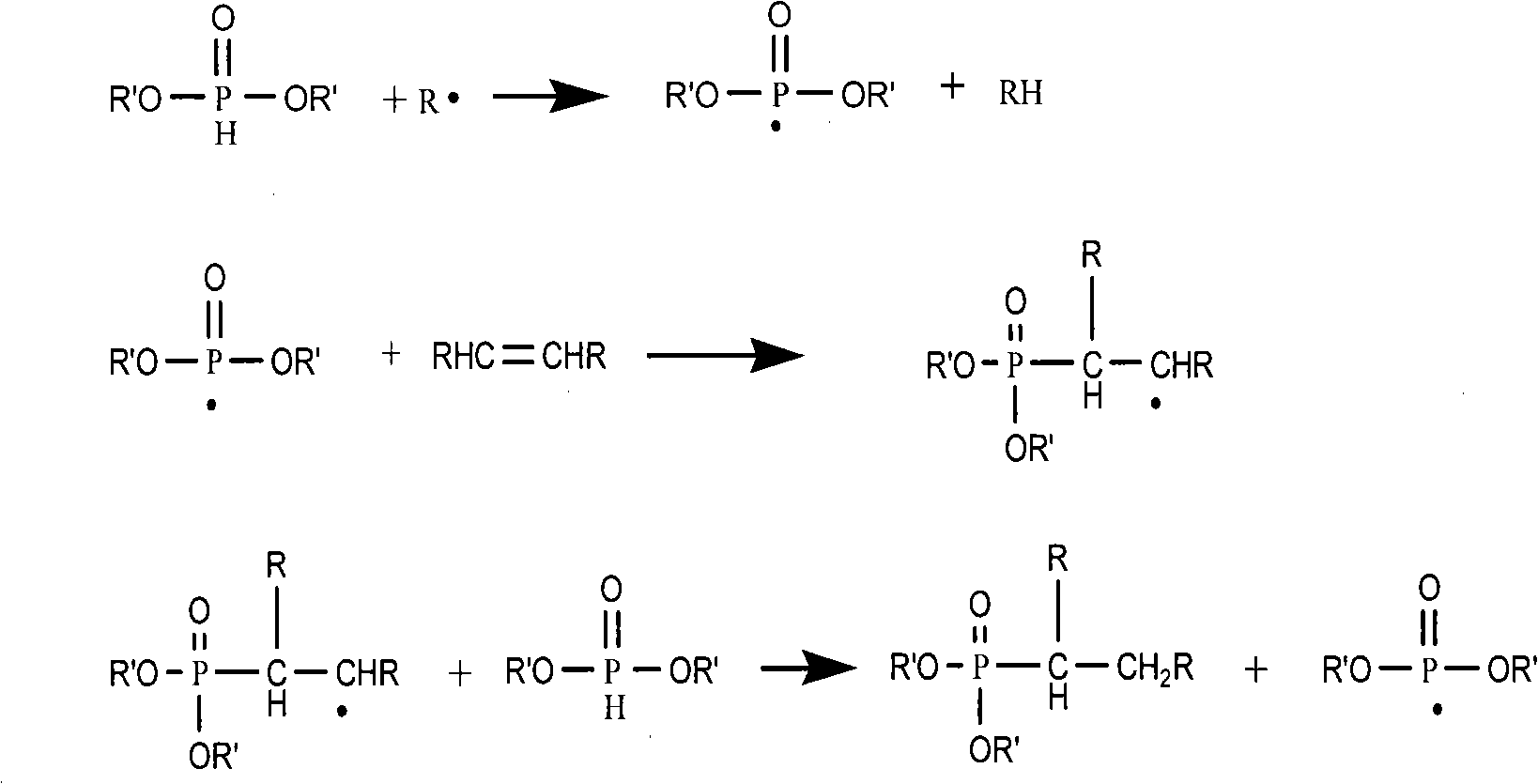
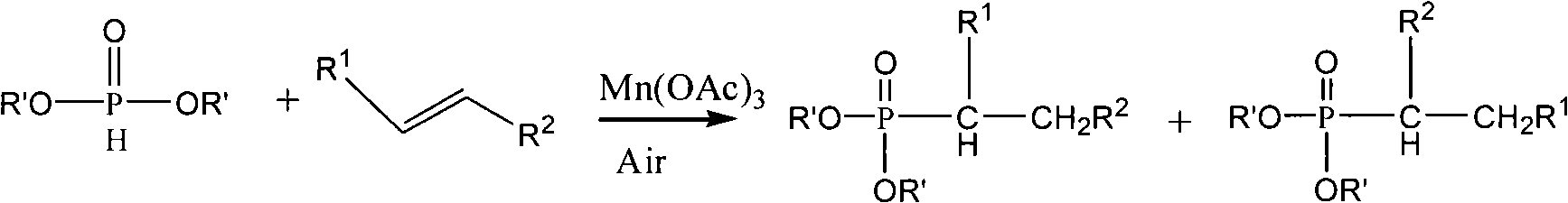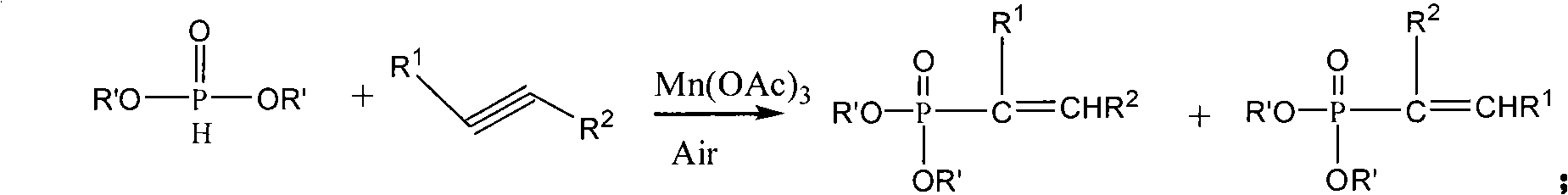Method for synthesis of organo-phosphines acid ester derivant
A technology of derivatives and phosphonates, applied in the field of synthesizing organic phosphonate derivatives, can solve the problems of poor method selectivity, poor selectivity, rare raw materials, etc., and achieve the effects of mild reaction conditions, improved selectivity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This example is the synthesis of 1,3-diphenyl-2-propen-1-one-2-phosphonic acid diethyl ester. With 1,3-diphenyl-2-propen-1-one and diethyl phosphite as raw materials, the reaction formula is as follows:
[0046]
[0047] The preparation method is:
[0048] (1) Add 1mmol of 1,3-diphenyl-2-propen-1-one, 2mmol of diethyl phosphite, 5mL of acetic acid, and 3mmol of manganese (III) acetate in a 25mL round-bottomed flask, at 80 Stir at ℃ for 1-2 hours;
[0049] (2) TLC tracking reaction until complete completion;
[0050] (3) column chromatography separation after the end of the reaction, the target product was obtained with a yield of 73%;
[0051] The product is analyzed, the data are as follows:
[0052] H NMR spectrum (400MHz, CDCl 3 ): δ7.87-7.85 (d, 2H, ArH), 7.73 (d, 1H, J P-H =25.7Hz, CH), 7.40-7.09(m, 8H, ArH), 4.06(m, 4H, OC H 2 CH 3 ×2), 1.16(t, 6H, 3 J H-H =5.7, OCH 2 C H 3 ×2);
[0053] C NMR spectrum (100MHz, CDCl 3 ): δ190.5(d, 2 J C-P ...
Embodiment 2
[0056] This example is the synthesis of 1-(4-methoxy)phenyl-3-phenyl-2-propen-1-one-2-phosphonic acid diethyl ester. Using 1-(4-methoxy)phenyl-3-phenyl-2-propen-1-one and diethyl phosphite as raw materials, the reaction formula is as follows:
[0057]
[0058] The preparation method is:
[0059] (1) In a 25mL round bottom flask, add 1mmol of 1-(4-methoxy)phenyl-3-phenyl-2-propen-1-one, 2mmol of diethyl phosphite, 5mL of acetic acid, 3mmol of manganese (III) acetate, stirred at 80°C for 1-2 hours;
[0060] (2) TLC tracking reaction until complete completion;
[0061] (3) Column chromatography separation after the reaction was completed, and the target product was obtained with a yield of 78%;
[0062] The product is analyzed, the data are as follows:
[0063] H NMR spectrum (400MHz, CDCl 3 ): δ7.90(d, 2H, ArH), 7.73(d, 1H, 3 J P-H =26.1Hz, CH), 7.28(d, 2H, ArH), 7.19(m, 3H, ArH), 6.81(d, 2H, ArH), 4.12(m, 4H, OCH 2 ×2), 3.78(s, 3H, CH 3 ), 1.24(t, 6H, 3 J H-H = 6....
Embodiment 3
[0067] This example is the synthesis of 1,3-bis(4-methoxy)phenyl-2-propen-1-one-2-phosphonic acid diethyl ester. With 1,3-bis(4-methoxy)phenyl-2-propen-1-one and diethyl phosphite as raw materials, the reaction formula is as follows:
[0068]
[0069] The preparation method is:
[0070] (1) Add 1mmol of 1,3-bis(4-methoxy)phenyl-2-propen-1-one, 2mmol of diethyl phosphite, 5mL of acetic acid, and 3mmol of manganese acetate in a 25mL round bottom flask. (III), stirring at 80° C. for 1-2 hours;
[0071] (2) TLC tracking reaction until complete completion;
[0072] (3) column chromatography separation after the end of the reaction, the target product was obtained with a yield of 76%;
[0073] The product is analyzed, the data are as follows:
[0074] H NMR spectrum (400MHz, CDCl 3 ): δ7.90(d, 2H, ArH), 7.65(d, 1H, 3 J P-H =25.5Hz, CH), 7.24(d, 2H, ArH), 6.80(d, 2H, ArH), 6.66(d, 2H, ArH), 4.09(m, 4H, OCH 2 ×2), 3.77(s, 3H, CH 3 ), 3.67 (s, 3H, CH 3 ), 1.21(t, 6H, 3 J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com