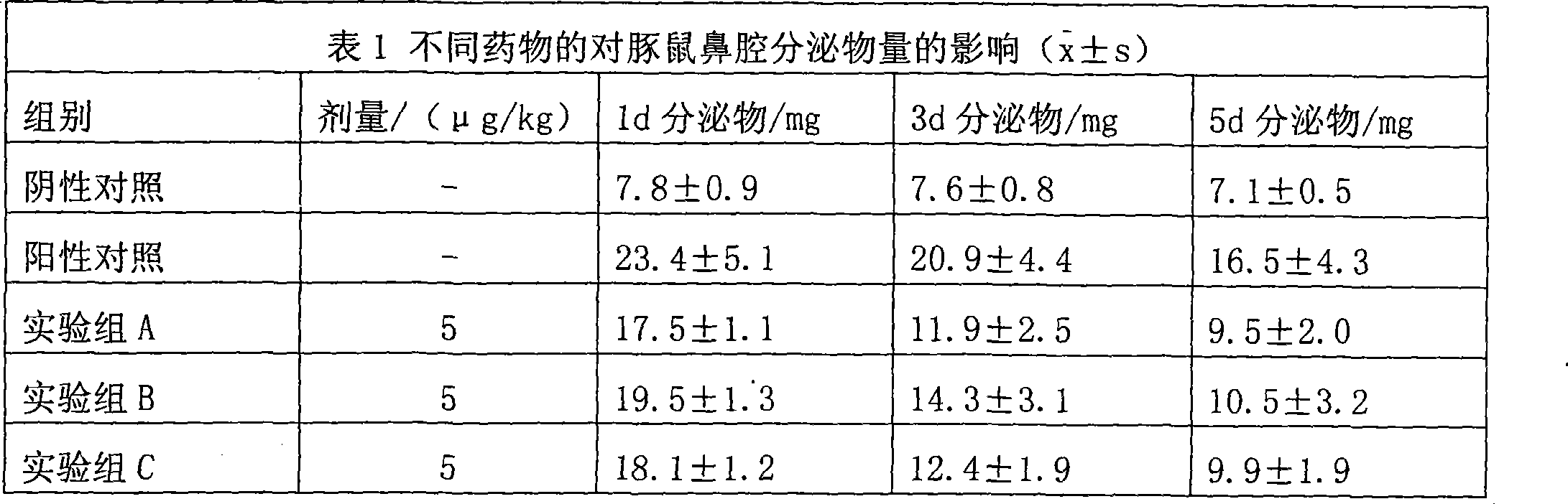
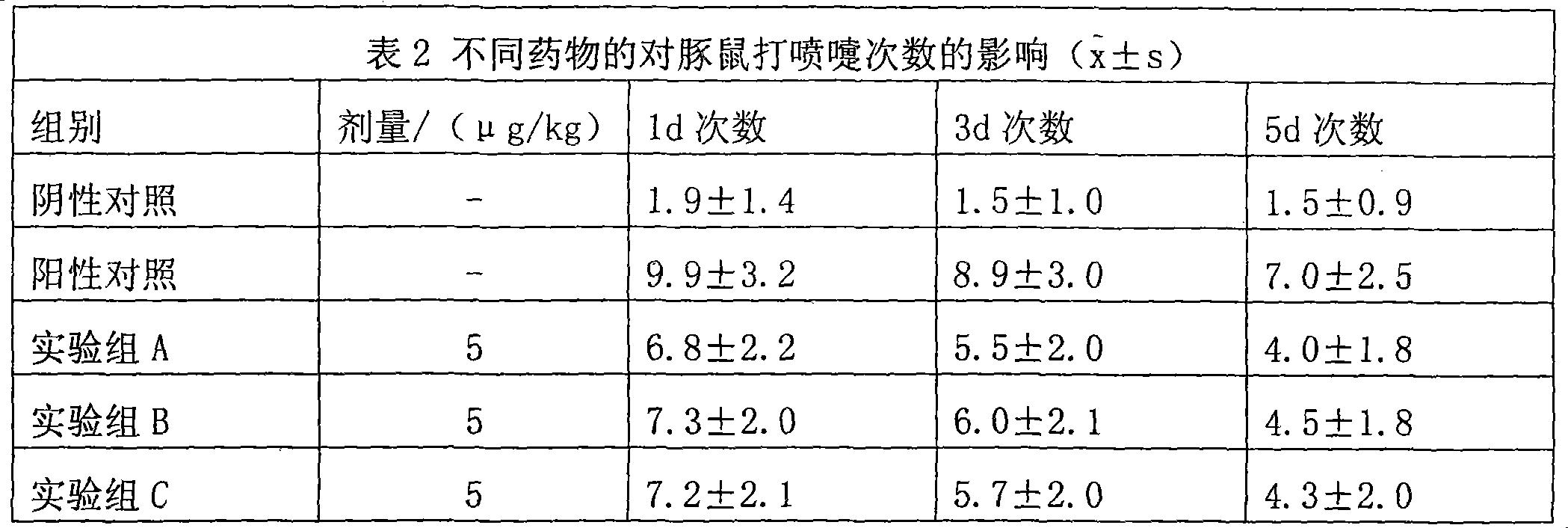
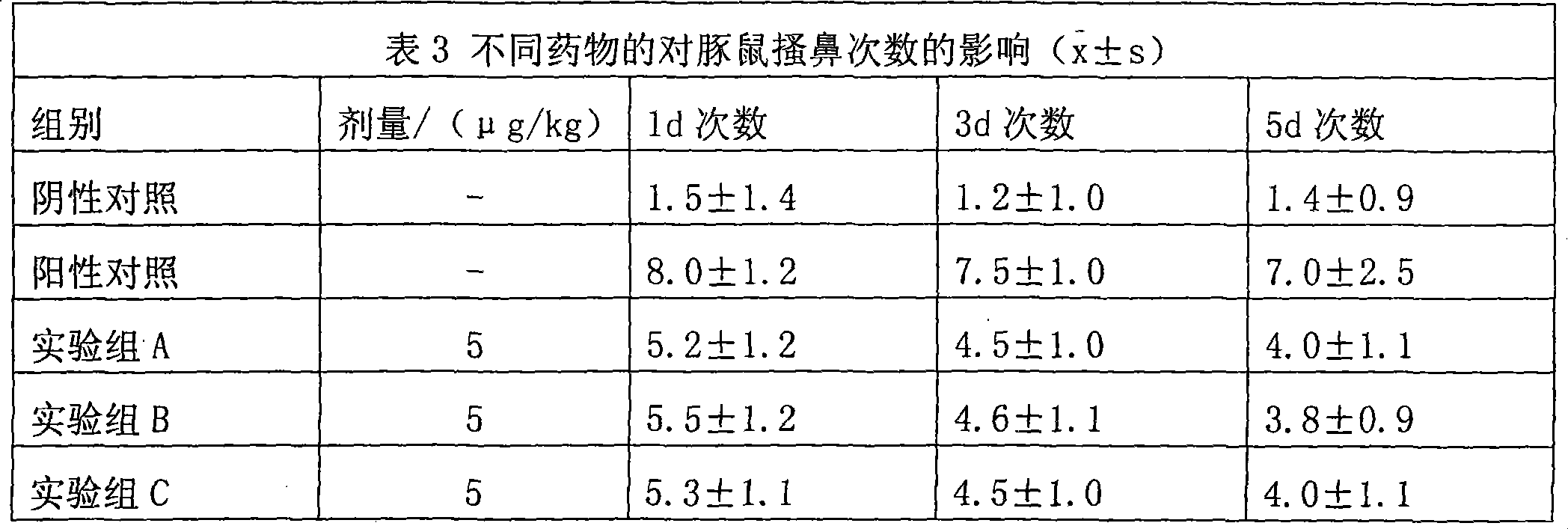
Uses of methylprednisolone and derivatives thereof in preparing medicament for treating allergic rhinitis
A technology for allergic rhinitis and methylprednisolone, which can be used in drug combination, drug delivery, and pharmaceutical formulations to solve problems such as telangiectasia, high price, and skin atrophy, so as to reduce systemic effects and avoid atrophic effects , the effect of reducing the therapeutic dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Methylprednisolone 20mg
[0056] Ephedrine Hydrochloride 0.5g
[0057] Glycerol 5ml
[0058] Carbomer 934 300mg
[0059] 5% ethylparaben ethanol solution 600μL
[0060] Get the prescribed amount of Carbomer 934 and add glycerin to wet and grind, add 50ml of purified water to swell as a gel matrix, and purify the prescribed amount of ephedrine hydrochloride, methylprednisolone, and 5% ethylparaben ethanol solution in 20ml Disperse with water, slowly add to the gel matrix and stir well, adjust the pH value to 6.0 to 7.0 with an appropriate amount of 1mol / L sodium hydroxide, and add purified water to 100ml.
Embodiment 2
[0062] The scheme described in embodiment 1, active ingredient is changed into:
[0063] Methylprednisolone Acetate 25mg
[0064] L-carbastine 20mg
[0065] The dosage of Carbomer 934 was adjusted to 200 mg, other ingredients and preparation method were the same as in Example 1, the prepared pharmaceutical composition was sub-packaged, and the nozzle was installed to obtain the nasal spray gel.
Embodiment 3
[0067] Methylprednisolone Acetone 50mg
[0068] Sodium carboxymethylcellulose 0.4g
[0069] Methylparaben 30mg
[0070] Glycerol 5ml
[0071] Take the prescribed amount of sodium carboxymethyl cellulose, add sodium chloride to 20ml of purified water, stir well and filter, add the prescribed amount of methylparaben into 50ml of purified water, heat to 70°C until completely dissolved, let cool and dissolve the two parts The solution is mixed and added with prescribed amount of methylprednisolone acetone, stirred evenly, the pH value is adjusted to 5.5 to 7.0, and the remaining water for injection is added. Get nasal drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com