Human beta-globin gene and recombinant adeno related viral vector thereof
A technology of β-globin and viral vectors, applied in the field of biomedicine, can solve the problems of small vector capacity, insufficient site-specific integration, and size limitation of target gene fragments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
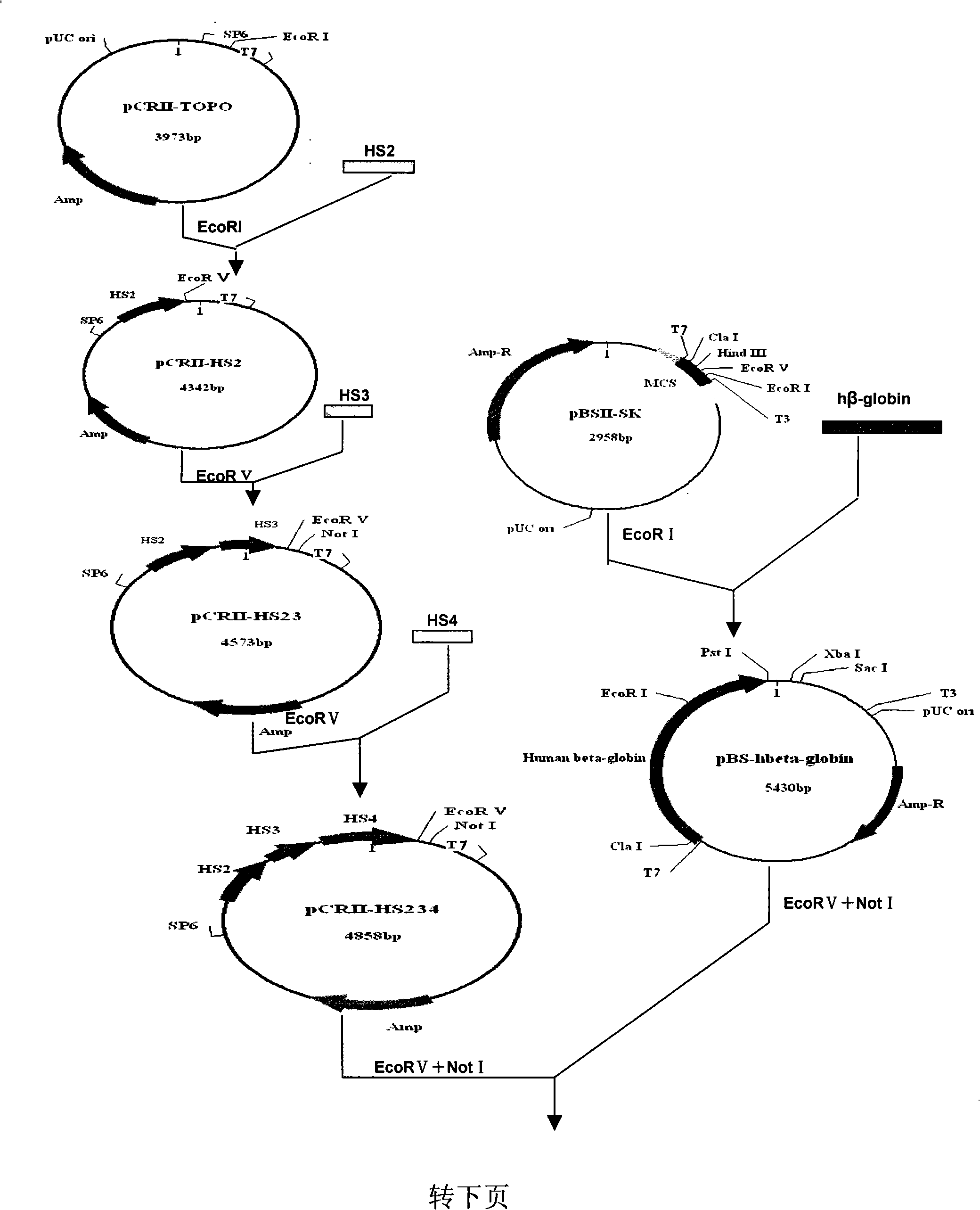
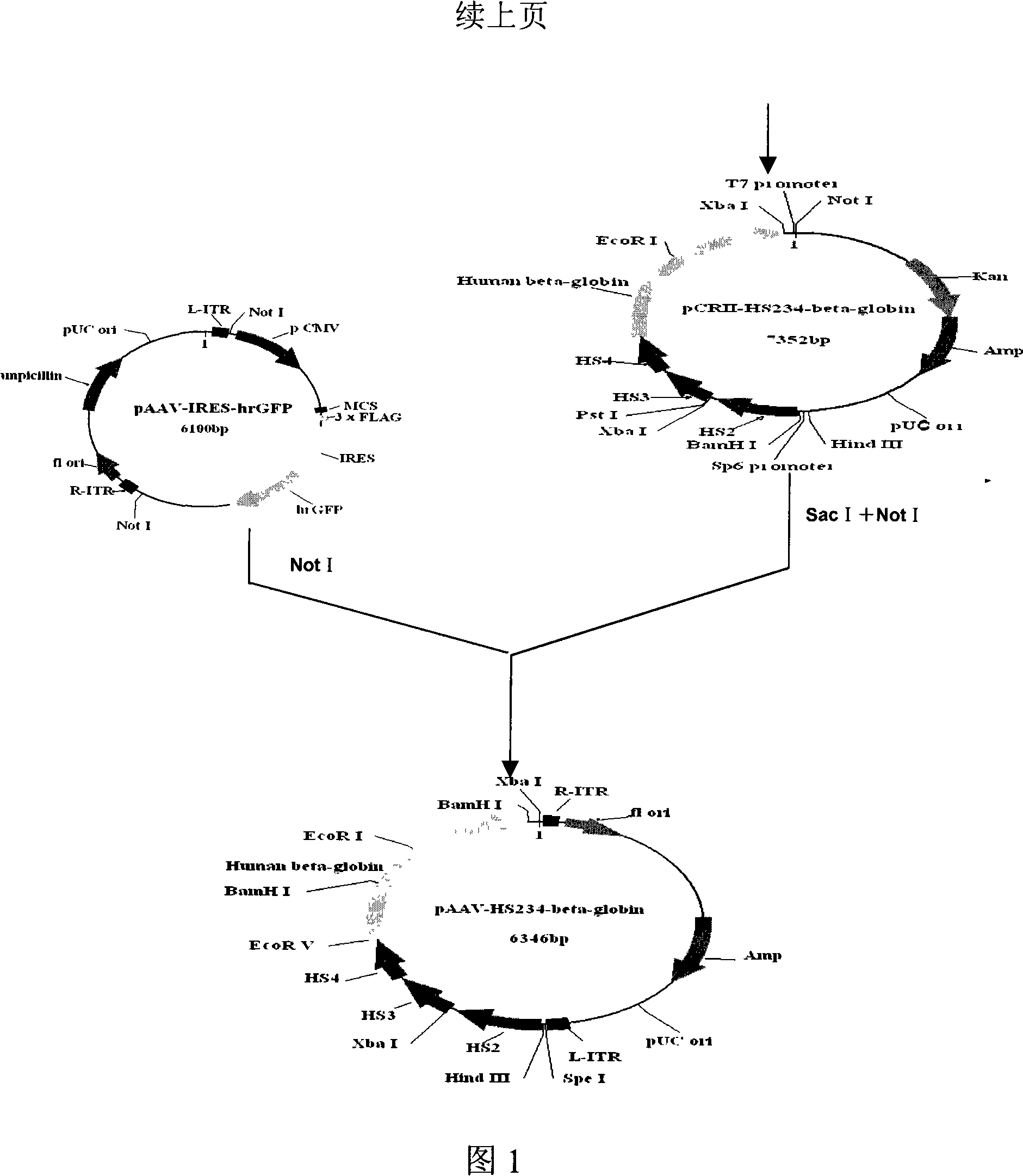
[0032] Example 1 Construction of recombinant adeno-associated virus vector pAAV-HS234-hβ-globin
[0033] (1) HS234 fragment:
[0034] According to the existing fragment sequences of HS2, HS3 and HS4 (Nucl Acid Res 1994.22: 1006-1011; EMBO J 1993.12: 1077-1085; Nucl Acid Res 1990.9: 2159-2167; Nucl Acid Res 1991.19: 1413-1419; PNAS 1995.92: 67 6732) designed primers ①, ② and ③ respectively.
[0035] Primer ①P5: 5'-ttaaataagcttcagtttttccttagttcc-3' (as shown in SEQ ID NO.5),
[0036] P3: 5'-tctagaatatgtcacattctgtct-3' (as shown in SEQ ID NO.6);
[0037] Primer ②P5: 5'-tggtgtgccagatgtgtctatcag-3' (as shown in SEQ ID NO.7),
[0038] P3: 5'- gatatc gctgctatgctgtgcctccccca-3' (as shown in SEQ ID NO.8);
[0039] EcoRV
[0040] Primer ③P5: 5'-ggaccccagtacacaagaggggacgca-3' (as shown in SEQ ID NO.9),
[0041] P3: 5'- gatatc ggaatgggaggggagagtctctg-3' (shown in SEQ ID NO. 10).
[0042] EcoRV
[0043] From the human β-globin gene Genomic DNA extracted from normal human periph...
Embodiment 2
[0053] Example 2 Obtaining of Purified Recombinant Adeno-Associated Virus Vector rAAV / β-globin
[0054] HEK293 cells were used as packaging cells, subcultured with DMEM medium and 10% newborn bovine serum, the cells grew to 80%-85% confluent, and the pAAV-HS234-β-globin prepared in Example 1 was purified by calcium phosphate co-precipitation method Co-transfect HEK293 cells with helper plasmids pAAV2-RC and pHelper, each plasmid 10μg / dish (10cm), take 5ml of 1.5mol / L CaCl 2 , add water to 20ml, mix well, add 2xBuffer A (50mM Hepes, 1.5mM Na 2 HPO 4 , 280mM NaCl, pH 7.05) 25ml, mix well and let it stand for 5 minutes, when a white cloud appears, the transfection of 293 cells is started, 2ml / dish, and continue to be placed in a carbon dioxide incubator with 5% CO 2 , 37°C and saturated humidity for 65-70 hours, collect the cells and store them in a -70°C refrigerator. Next, purify, take out the frozen cells from -70°C and put them in a 37°C water bath for 30 minutes, freeze a...
Embodiment 3
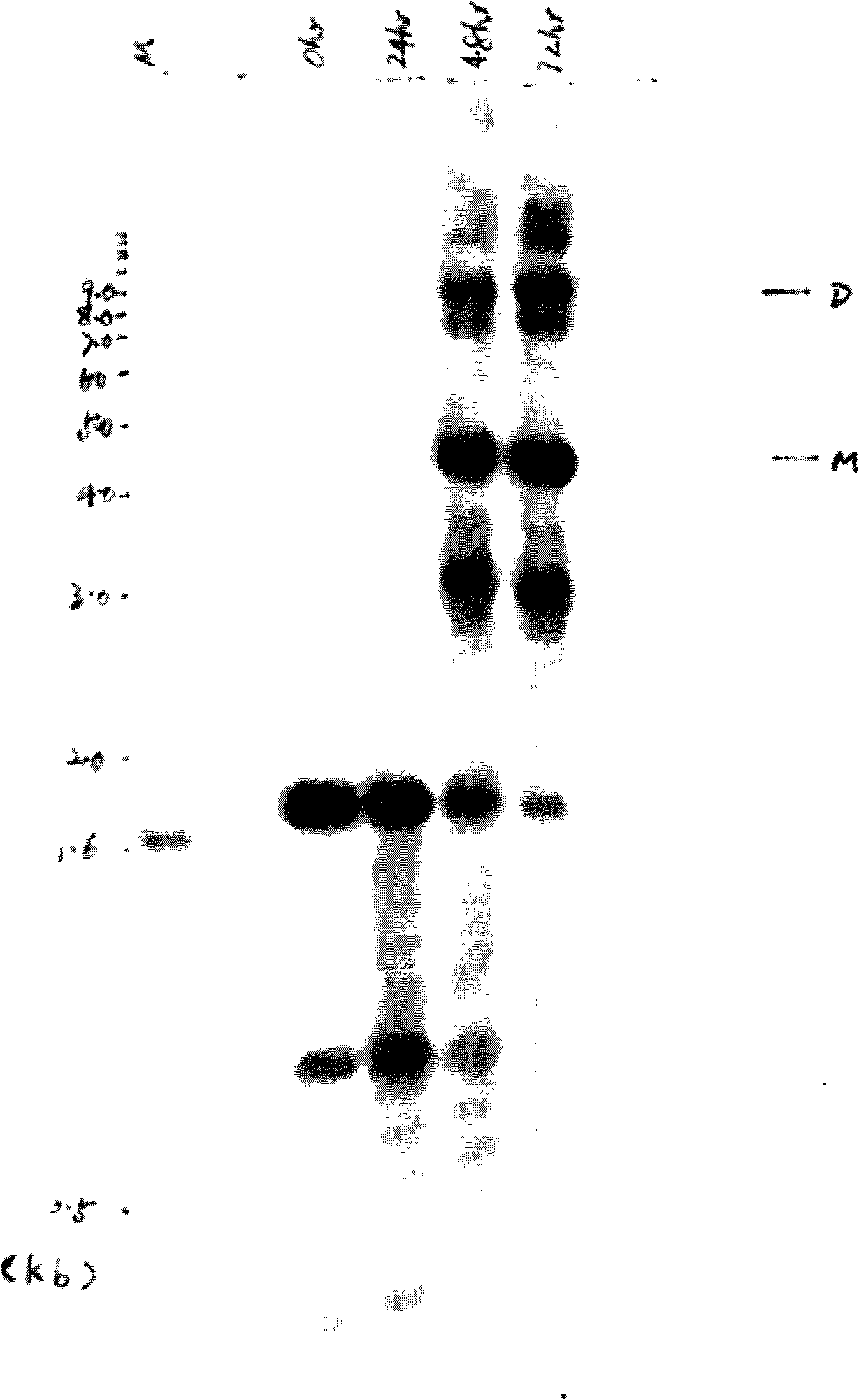
[0056] Example 3 Detection of the expression of the introduced exogenous β-globin gene in K562 cells by PCR and RT-PCR
[0057] Under sterile conditions, take 12×10 6K562 cells suspended in RPMI 1640 culture medium containing 10% newborn bovine serum and growing well were divided into control groups without pretreatment (8×10 6 cells) and group pretreated with hydroxyurea (4×10 6 Cells), the unpretreated control group added an appropriate amount of solution 1×PBS for dissolving hydroxyurea; 2 , 37°C, and cultured in saturated humidity for 4 hours, collect the cells respectively, and wash the cells once with 1×PBS. 16 μl of RPMI 1640 culture solution was added to the control group, and 16 μl (equivalent to 50 MOI) of the recombinant adeno-associated virus vector rAAV / β-globin obtained in Example 2 were added to the experimental group and the pretreatment group, and cultured under the above conditions, every 10- Shake once every 15 minutes. After 2 hours of transfection, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com