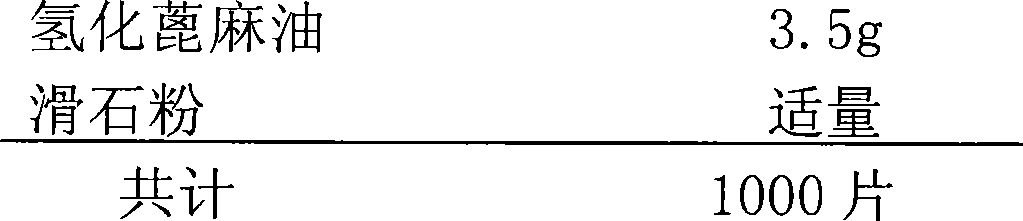Novel medicament composition for resisting thrombosis
An antithrombotic and composition technology is applied in the field of antithrombotic pharmaceutical compositions and their preparation, which can solve problems such as limitations and achieve the effects of reducing irritation, improving medication safety, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The drug compatibility test of embodiment 1 clopidogrel and indobufen
[0048] The purpose of this experiment is to verify whether the two drugs, clopidogrel and indobufen, are physically and chemically compatible. The two drugs, clopidogrel and indobufen, were prepared and stored in three different temperature and humidity environments, namely 25°C / 10%RH; 30°C / 60%RH; 40°C / 75%RH. Drugs were divided into a mixed group and an unmixed group as controls, which were respectively placed at these three temperatures. Samples were evaluated on day 1, day 2, day 7, day 14, day 30 for color, physical properties, chemical stability (by chromatography), and activity of each active ingredient under all storage conditions The degree of recovery (% mixed group / % control group) was 94.3%-103.7% for clopidogrel and 96.2%-104.4% for indobufen. During the observation, there was no obvious difference between the mixing group and the control group in the analysis of physical shape and chem...
Embodiment 2
[0050] Example 2 Compound Tablet Containing Clopidogrel 25mg and Indobufen 100mg
[0051] 1) Prescription:
[0052]
[0053]
[0054] 2) Preparation method: powder direct compression method
[0055] Mix 25g of clopidogrel, 40g of compressible starch, 40g of β-anhydrous lactose and 0.7g of anhydrous colloidal silicon dioxide; mix 100g of indobufen, 40g of β-anhydrous lactose and 40g of microcrystalline cellulose. Add hydrogenated castor oil and talcum powder, mix thoroughly together, pass through a 12-mesh nylon sieve for granulation, after the content of the granules is qualified, punch the granule powder with a 12mm punch and directly compress it into tablets to obtain the product.
Embodiment 3
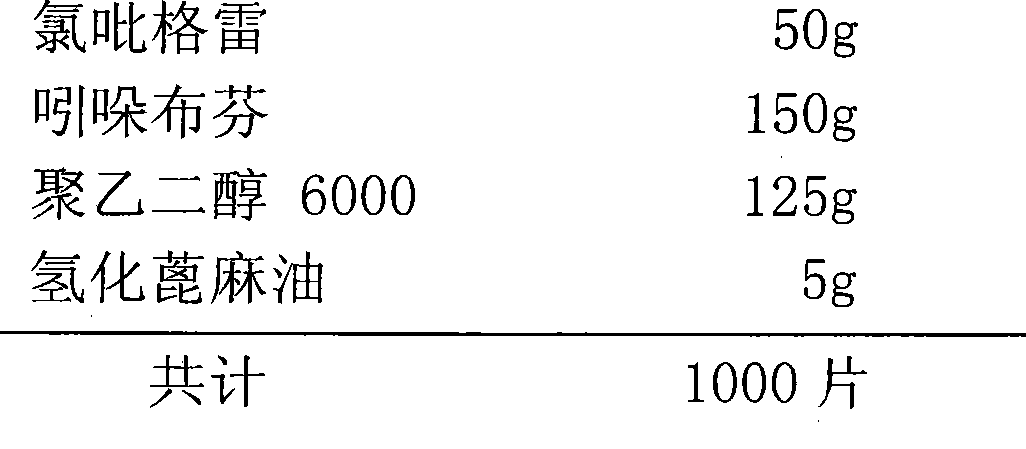
[0056] Example 3 Compound Tablet Containing Clopidogrel 50mg and Indobufen 150mg
[0057] 1) Prescription:
[0058]
[0059] 2) Preparation method: powder direct compression method
[0060] Stir clopidogrel, indobufen and macrogol6000 in a rapid mixing granulator for 15 minutes and mix evenly; add hydrogenated castor oil, continue stirring for 5 minutes to mix evenly, pass through a 12-mesh nylon sieve for granulation. After the granules pass the content determination, the final mixture powder is directly compressed into tablets according to the mass of the unit dose to obtain about 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com