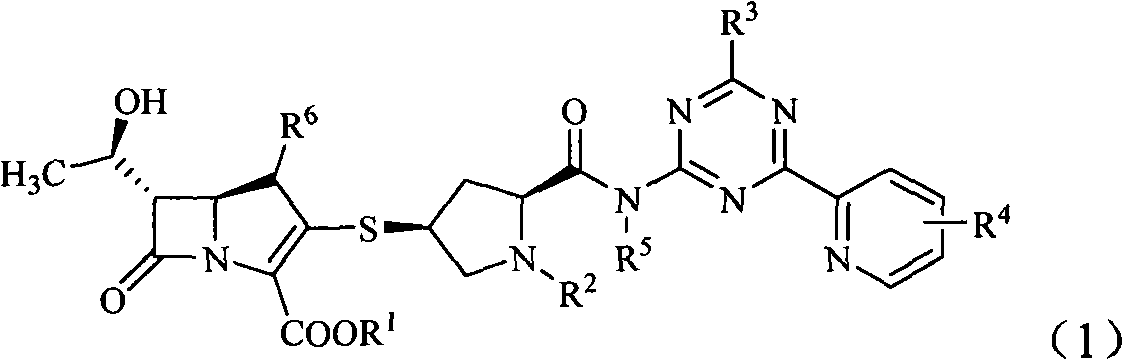
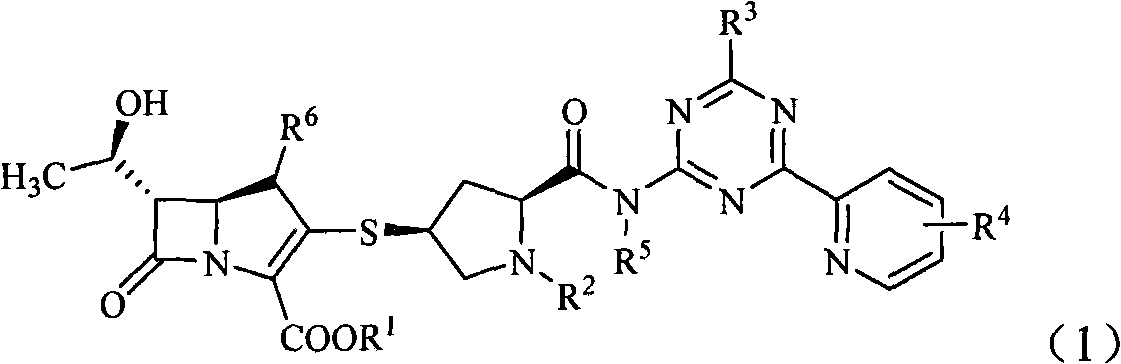
Penem derivative containing sulfhydryl pyrrolidine formamido triazine
A technology of compounds and hydrates, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, antibacterial drugs, etc., can solve the problems of low clinical utilization and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 (2S, 4S)-4-mercapto-2-formyl[[4-thiomethyl-6-(pyridin-2-yl)-1,3,5-triazin-2-yl]amino] -1-(tert-D Oxycarbonyl) pyrrolidine preparation
[0089] Add 8.7 g (30 mmol) of (2S,4S)-4-acetylthio-2-carboxy-1-(tert-butoxycarbonyl)pyrrolidine and 100 ml of anhydrous tetrahydrofuran into the dry reaction flask. Under nitrogen protection, 6.5g (40mmol) of 1,1-carbonyldiimidazole was added at room temperature, reacted for 0.5h, and 4.8g (50mmol) of 2-amino-4-thiomethyl-6-(pyridine- 100ml of a tetrahydrofuran solution of 2-yl)-1,3,5-triazine, and continue the reaction for 0.5h. Then 40ml of 1mol / l hydrochloric acid was added dropwise, extracted with ethyl acetate (50ml×2), the organic phase was washed with water and saturated sodium chloride solution successively, concentrated under reduced pressure, the residue was added with 100ml of 3mol / l hydrochloric acid, stirred for 2h, and The dilute alkaline solution was adjusted to be alkaline, and a solid was precipitated, w...
Embodiment 2
[0090] Example 2 (4R, 5S, 6S)-3-[(2S, 4S)-2-formyl[[4-thiomethyl-6-(pyridin-2-yl)-1,3,5-triazine -2-yl]amine Base]-1-(tert-butoxycarbonyl)pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo- [3,2,0]hept-2- Preparation of ene-2-carboxylic acid p-nitrobenzyl ester
[0091] In a dry reaction flask, add (4R, 5S, 6S)-3-diphenoxyphosphoryloxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-nitrogen Heterobicyclo-[3,2,0]hept-2-ene-2-carboxylic acid p-nitrobenzyl ester 35.8g (60mmol) in 400ml of acetonitrile solution was cooled to below -20°C, added diisopropylethylamine 16ml and (2S,4S)-4-Mercapto-2-formyl[[4-thiomethyl-6-(pyridin-2-yl)-1,3,5-triazin-2-yl]amino]-1- A solution of 29.1 g (65 mmol) of (tert-butoxycarbonyl)pyrrolidine in 200 ml of acetonitrile was stirred at 0°C for 24 h. After the reaction was completed, it was diluted with 600 ml of ethyl acetate, washed with water and saturated brine successively, the organic layer was dried and concentrated to ...
Embodiment 3
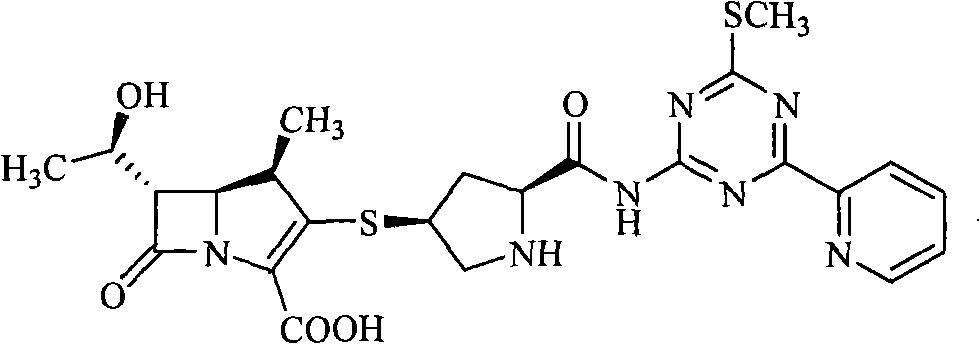
[0092] Example 3 (4R, 5S, 6S)-3-[(2S, 4S)-2-formyl[[4-thiomethyl-6-(pyridin-2-yl)-1,3,5-triazine -2-yl]amino]- Pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo-[3,2,0]hept-2- Preparation of ene-2-carboxylic acids
[0093](4R, 5S, 6S)-3-[(2S, 4S)-2-formyl[[4-thiomethyl-6-(pyridin-2-yl)-1,3,5-triazine-2 -yl]amino]-1-(tert-butoxycarbonyl)pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-nitrogen Heterobicyclo-[3,2,0]hept-2-ene-2-carboxylic acid p-nitrobenzyl ester 23.8g (30mmol) was dissolved in 100ml of dichloromethane, added anisole 30ml and nitromethane 30ml, in Add 200ml of nitromethane solution of 1mol / L aluminum chloride dropwise at -50°C, stir at -30°C for 4h, add 300ml of water, precipitate a solid, filter, dissolve the filter cake in a mixture of 600mlTHF and 50ml of water, add 10% palladium-carbon 5g, stirred at room temperature under 5MPa hydrogen pressure for 2h, filtered palladium-carbon, added THF 200ml to the filtrate, sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com