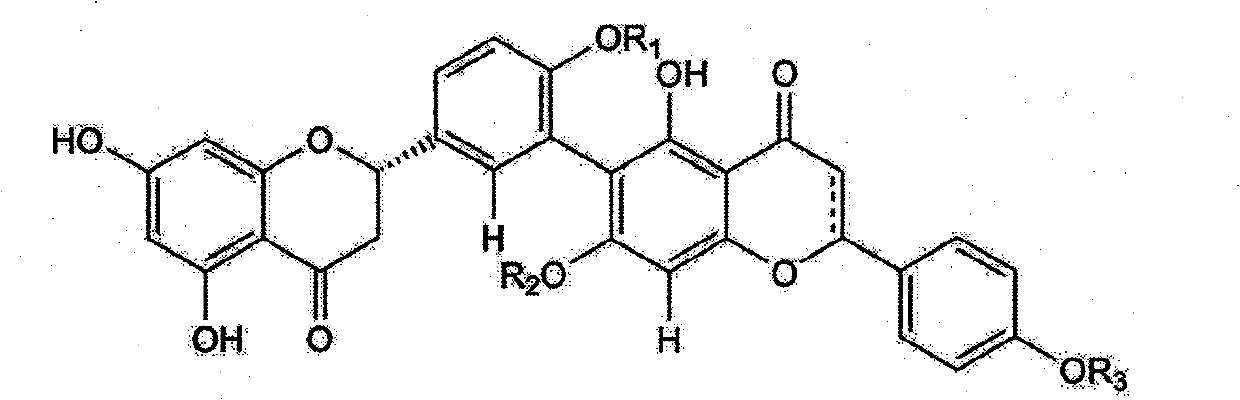
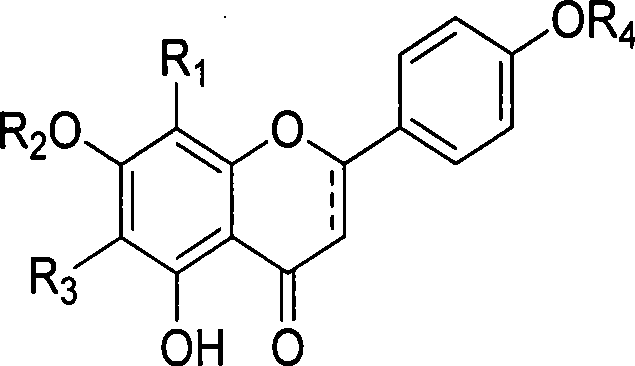
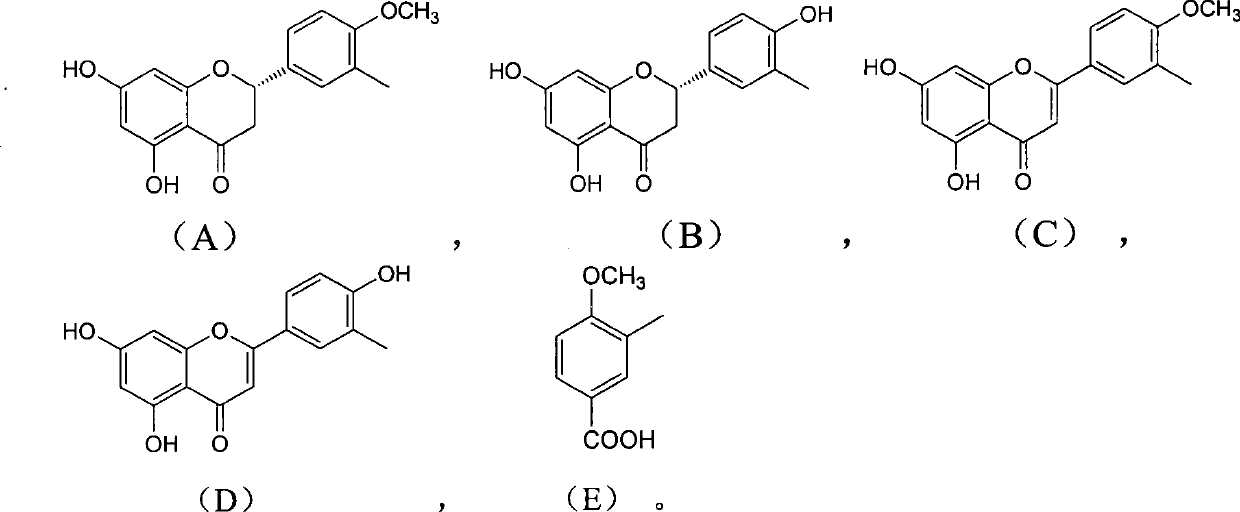
Use and preparation method of flavonoid derivatives
A technology of derivatives and flavonoids, which is applied in the directions of medical preparations containing active ingredients, food preparation and application, can solve problems such as loss of consciousness and damage, and achieve the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Pulverize 4.2 kg of dried whole herb of Selaginella uncinata (Desv.) Spring, extract by heating and refluxing three times with 60% ethanol 10 times the weight of Selaginella uncinata (Desv.) Spring, 2 hours each time, and combine Extraction solution;
[0044] (2) Remove insoluble matter by filtration, and dry the filtrate under reduced pressure to obtain ethanol extract.
[0045] (3) The ethanol extract was extracted 3 times with equal volumes of ethyl acetate and n-butanol respectively;
[0046] (4) Take the ethyl acetate extract and dry it under reduced pressure in vacuo to obtain the ethyl acetate extract.
[0047](4) The ethyl acetate extract was subjected to open silica gel column chromatography, and the chloroform:methanol ratios were 99:1, 98:2, 97:3, 95:5, 9:1, 8:2, 7:3 , 6:4 solvent gradient elution. Using comprehensive separation methods such as gel Sephadex LH-20 column chromatography, ODS column chromatography, and RP-18 high-performance liquid phase ...
Embodiment 2
[0052] Compounds 1-16 prepared in Example 1 were formulated into liquids with a concentration of 180 μmol / L, 90 μmol / L, and 45 μmol / L respectively as the drug group, and 10% DMSO was used as the control group to protect PC12 cells from hypoxia. test, and calculate the survival percentage of PC12 cells. The experimental results were calculated by Student's ttest, expressed as: mean ± SD. The results are shown in Table 1.
[0053] Effects of compounds in table 1 on PC12 cell hypoxic injury (x ± SD)
[0054]
[0055] Note: Compared with the control group * P** P<0.01
[0056] Conclusion: It can be seen from Table 9 that compared with the blank solvent group, compound 1-16 has a strong protective effect on hypoxic PC12 cells at different concentrations, and has anti-hypoxic activity in vitro. It shows that these 16 compounds can be used as anti-hypoxic drugs.
Embodiment 3
[0058] The compounds 1-16 prepared in Example 1 were formulated into liquids with a concentration of 40 mg / mL as the drug group, and 20% DMSO was used as the control group to carry out the airtight hypoxic endurance test in mice, and observe the mice in the airtight condition. The following survival time. The results are shown in Table 2.
[0059] Survival of mice under table 2 hypoxic conditions (x±SD, n=10)
[0060]
[0061] Note: Compared with the control group * P** P<0.01
[0062] Conclusion: As can be seen from Table 10, compared with the control group, compound 1-16 can significantly improve the survival time of mice under airtight hypoxic conditions, has certain anti-hypoxic activity in vivo, and can be used as an anti-hypoxic drug .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com