Sertindole synthesis method
A synthesis method and technology of serindole, applied in the field of serindole synthesis, can solve problems such as high cost and long hydrogenation time, and achieve the effects of low cost, short reaction time and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
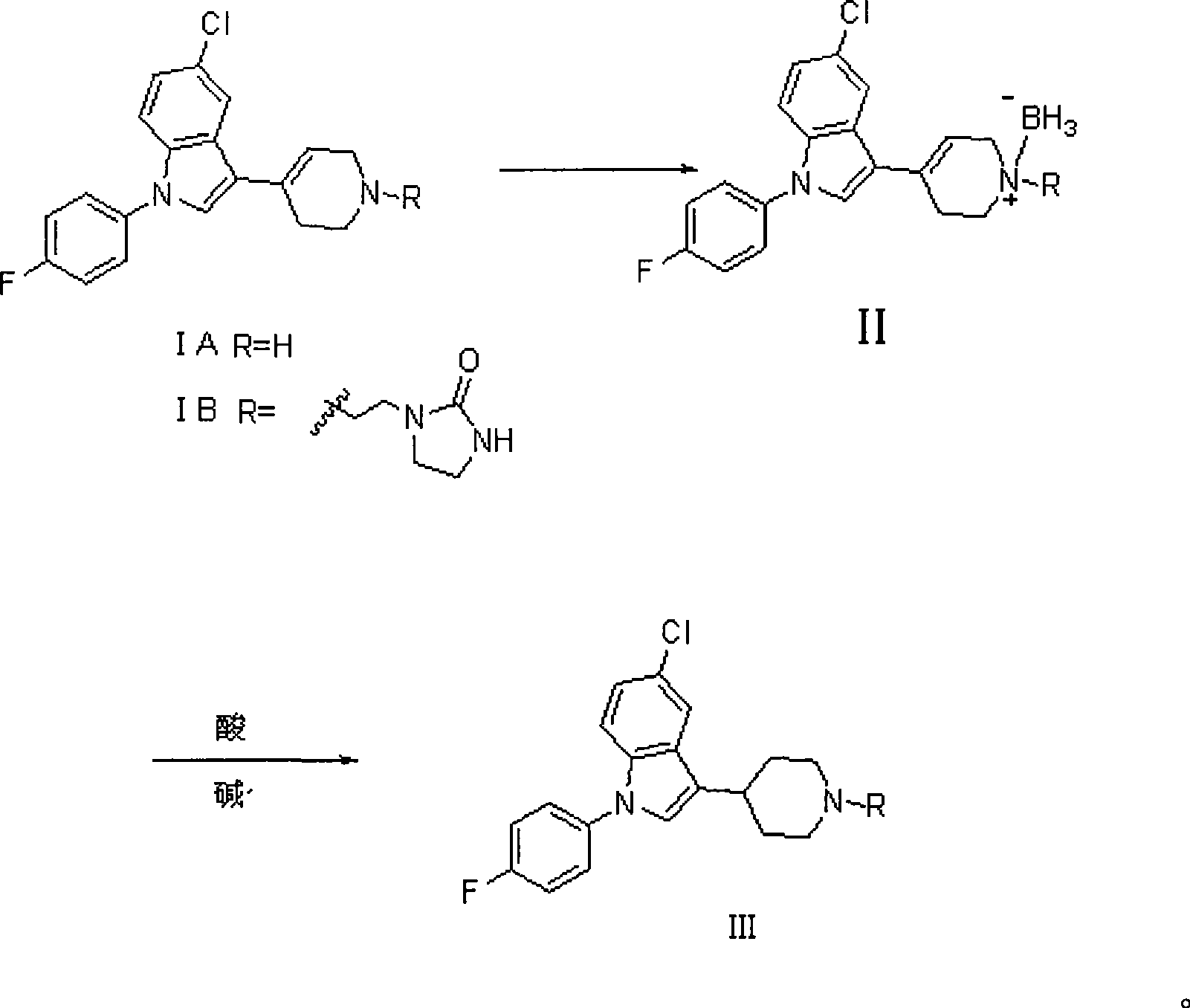
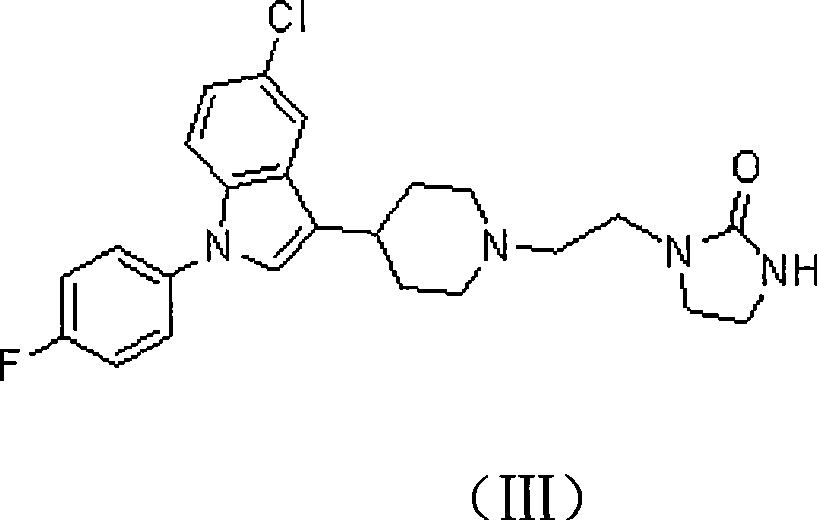
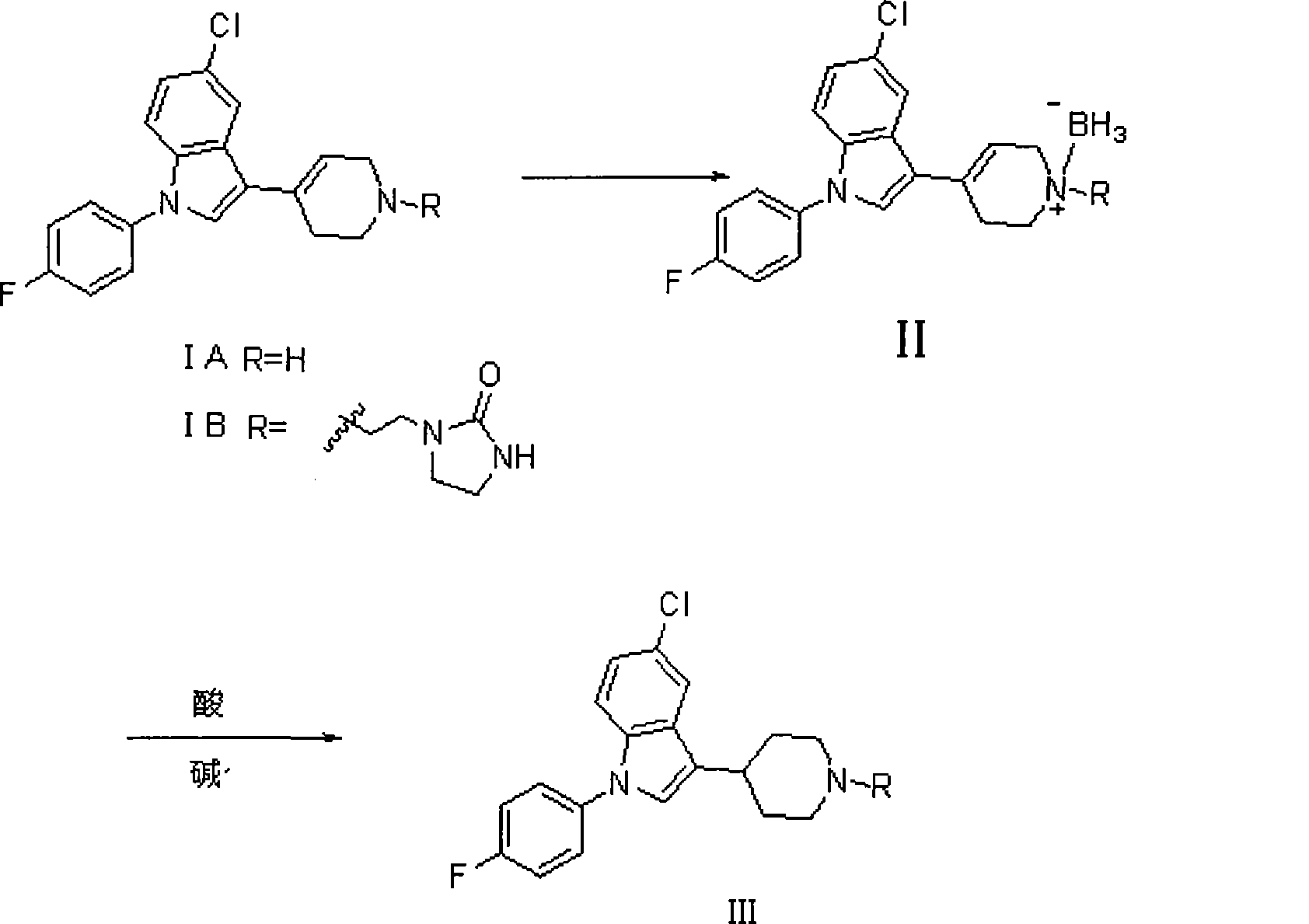
[0031] 1-[2-[4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl]-5,6-dihydropiperidin-1(2H)-yl] —2-imidazolinone hydrochloride (3g, 0.0063mol) is placed in 50ml tetrahydrofuran, stir well, add NaBH 4 (1.44g, 0.038mol);
[0032] Then, at 30°C, 0.25ml of glacial acetic acid was added dropwise and stirred for 30min.
[0033] Then, at room temperature, 7.5 ml of HCl with a weight concentration of 37% was added dropwise to the reaction solution, and stirred for 30 min;
[0034] Then add 50ml of water to the reaction solution, evaporate tetrahydrofuran (THF), adjust the pH to 12 with a 30% NaOH solution by weight, extract 3 times with dichloromethane, combine the extracts, backwash once with saturated NaCl solution, with anhydrous Na 2 SO 4 Dry, filter, and evaporate to dryness to obtain 2.5 g of a white solid, which is the target product, with a yield of 89.9%, m.p.153-154°C. 1 HNMR (400MHz, CDCl3): δ1.87 (dt, 2H), 2.05 (broad d, 2H), 2.27 (t, 2H), 2.62 (t, 2H), 2.82 (tt, 1H), 3.13 ...
Embodiment 2
[0036] 1-[2-[4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl]-5,6-dihydropiperidin-1(2H)-yl] —2-imidazolinone (1.25g, 0.0028mol) is placed in 15ml 2-methyltetrahydrofuran, stirred evenly, and NaBH is added at room temperature 4 (0.22g, 0.057mol), at 20°C, 0.5ml of glacial acetic acid (0.084mol) was added dropwise, and stirred at room temperature for 30min. Then, at room temperature, 4 ml of HCl with a weight concentration of 35% was added dropwise to the reaction solution, and stirred for 30 min. Alkalization with 20% NaOH solution by weight, and the others were the same as in Example 1 to obtain 0.66 g of white solid with a yield of 52.4%. Melting point and spectral data are the same as above.
Embodiment 3
[0038] The raw material 1-[2-[4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl]-5,6-dihydropiperidin-1(2H)-yl ]—2-imidazolinone hydrochloride (0.3g, 0.63mmol) was placed in 8ml tetrahydrofuran, stirred evenly, and KBH was added at room temperature 4(0.21g, 3.79mmol), below 30°C, add 0.07ml of glacial acetic acid dropwise, and stir at room temperature for 30min. At room temperature, 0.8 ml of sulfuric acid with a weight concentration of 60% was slowly added dropwise to the reaction liquid, and stirred for 30 min. Others were the same as in Example 1, and evaporated to dryness to obtain a white solid, which was separated by column chromatography to obtain 120 mg of the product with a yield of 43.16%. Melting point and spectral data are the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com