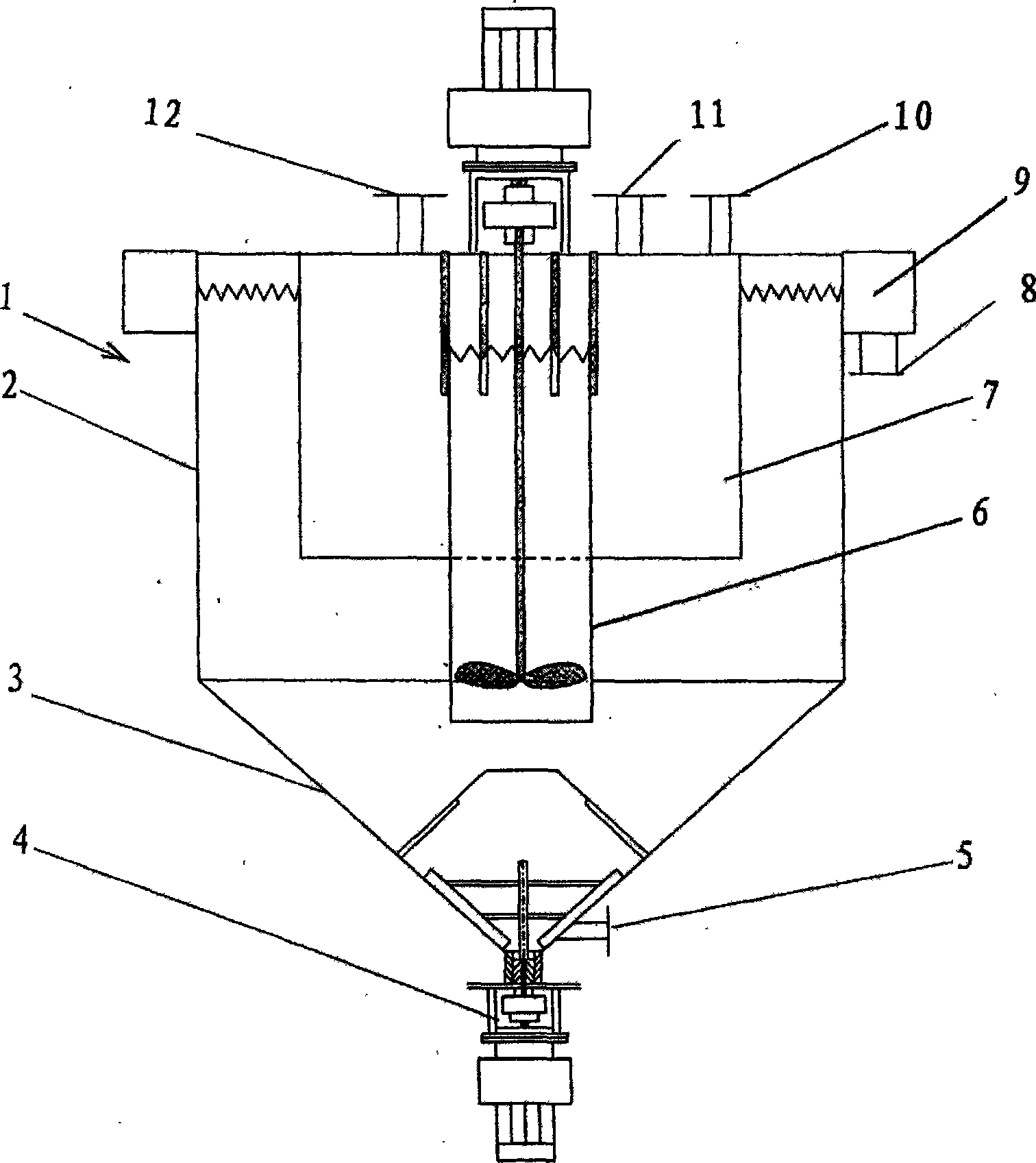
Method for preparing calcium sulphate with sulfate containing bittern and calcium containing bittern
A technology of calcium sulfate dihydrate and sulfate, applied in the direction of calcium/strontium/barium sulfate, etc., can solve problems such as environmental pollution, waste of calcium sulfate resources, etc., and achieve the effects of simple process, reduced entrainment, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
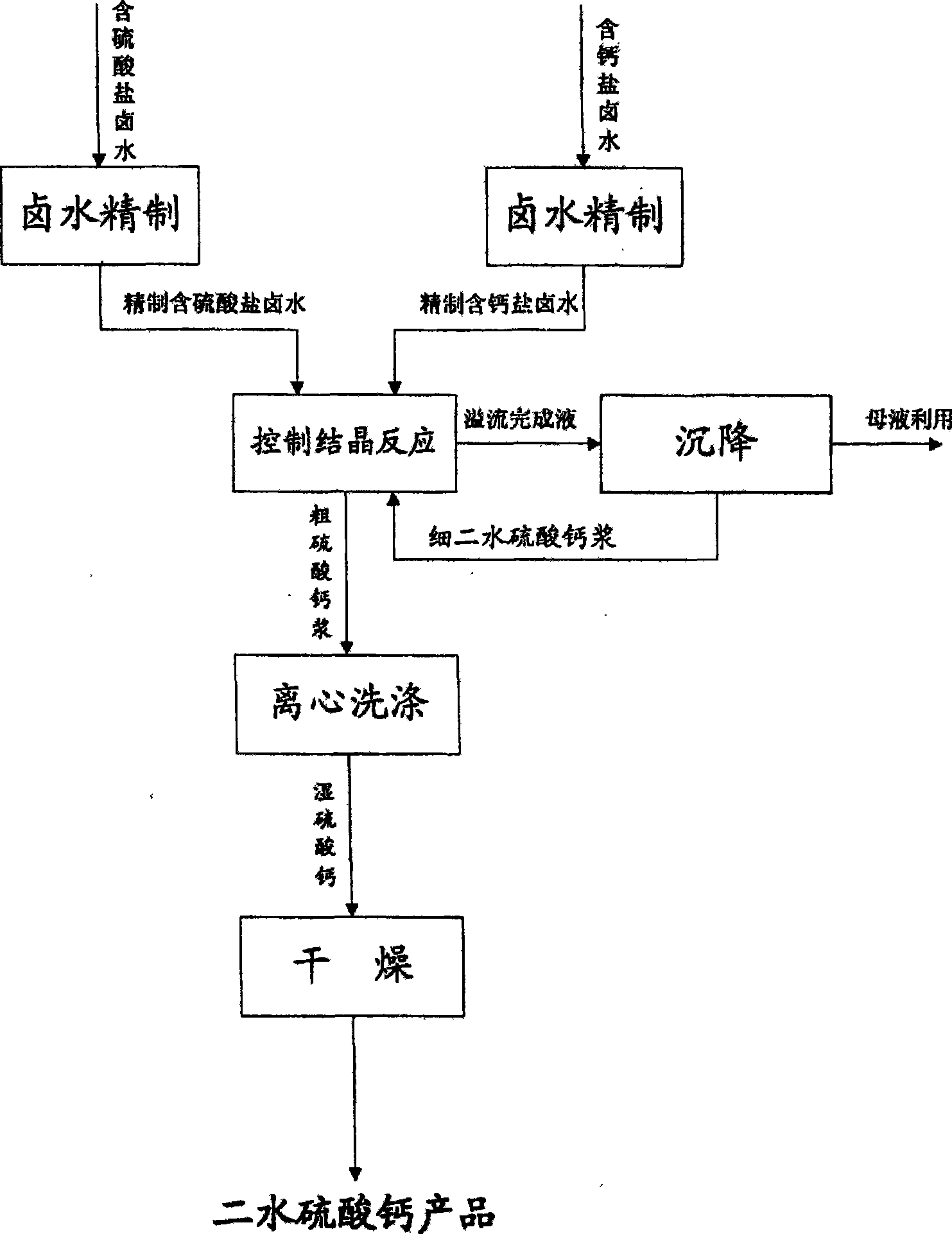
[0039] Take sulfate-containing brine and calcium salt-containing brine, first use a settler to settle it to remove part of the insoluble matter, and discharge it from the lower part of the settler. The upper liquid phase overflowed from the settler is pumped into the plate and frame filter press, and the liquid phase is refined sulfate-containing brine and refined calcium-containing salt brine. The chemical composition of the refined sulfate-containing brine is detected as: K + 13g / l, Na + 60.7g / l, Mg 2+ 51.74g / l, SO 4 2- 64g / l, Cl - 175g / l; the chemical composition of the refined brine containing calcium salt is: K + 13g / l, Na + 60.7g / l, Ca 2+ 85.17g / l, Cl - 198.6g / l.
[0040] The sedimentation refined sulfate-containing brine and calcium salt-containing brine are added to a controlled crystallization reactor for reaction crystallization.
[0041] First, according to the SO in refined sulfate-containing brine and refined calcium-containing brine 4 2- : Ca 2+ The ...
Embodiment 2
[0048] Take sulfate-containing brine and calcium salt-containing brine, first use a settler to settle it to remove part of the insoluble matter, and discharge it from the lower part of the settler. The upper liquid phase overflowed from the settler is pumped into the plate and frame filter press, and the liquid phase is refined sulfate-containing brine and refined calcium-containing salt brine. The chemical composition of the refined sulfate-containing brine is detected as: K + 13g / l, Na + 60.7g / l, Mg 2+ 48.20g / l, SO 4 2- 50g / l, Cl - 175g / l; the chemical composition of the refined brine containing calcium salt is: K + 13g / l, Na + 60.7g / l, Ca 2+ 60g / l, Cl - 180.46g / l.
[0049] The sedimentation refined sulfate-containing brine and calcium salt-containing brine are added to a controlled crystallization reactor for reaction crystallization.
[0050] First, according to the SO in refined sulfate-containing brine and refined calcium-containing brine 4 2- : Ca 2+ The ra...
Embodiment 3
[0057] Take sulfate-containing brine and calcium salt-containing brine, first use a settler to settle it to remove part of the insoluble matter, and discharge it from the lower part of the settler. The upper liquid phase overflowed from the settler is pumped into the plate and frame filter press, and the liquid phase is refined sulfate-containing brine and refined calcium-containing salt brine. The chemical composition of the refined sulfate-containing brine is detected as: Na + 116.7g / l, SO 4 2- 80g / l, Cl - 121.30g / l; the chemical composition of the refined brine containing calcium salt is: Na + 51.83g / l, Ca 2+ 85g / l, Cl - 230.66g / l.
[0058] The sedimentation refined sulfate-containing brine and calcium salt-containing brine are added to a controlled crystallization reactor for reaction crystallization.
[0059] First, according to the SO in refined sulfate-containing brine and refined calcium-containing brine 4 2- : Ca 2+ The ratio of the molar ratio of 1:1 is tha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com