Method for preparation of high-purity fatty acyl amino acid salt
A technology of fatty acyl amino acid salt and fatty acyl amino acid, which is applied in the field of preparation of high-purity fatty acyl amino acid salt, can solve the problems of equipment corrosion and environmental protection pressure, easy formation of viscous paste, affecting product mildness and performance, etc., and achieves impurity residue Less, stable product quality, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
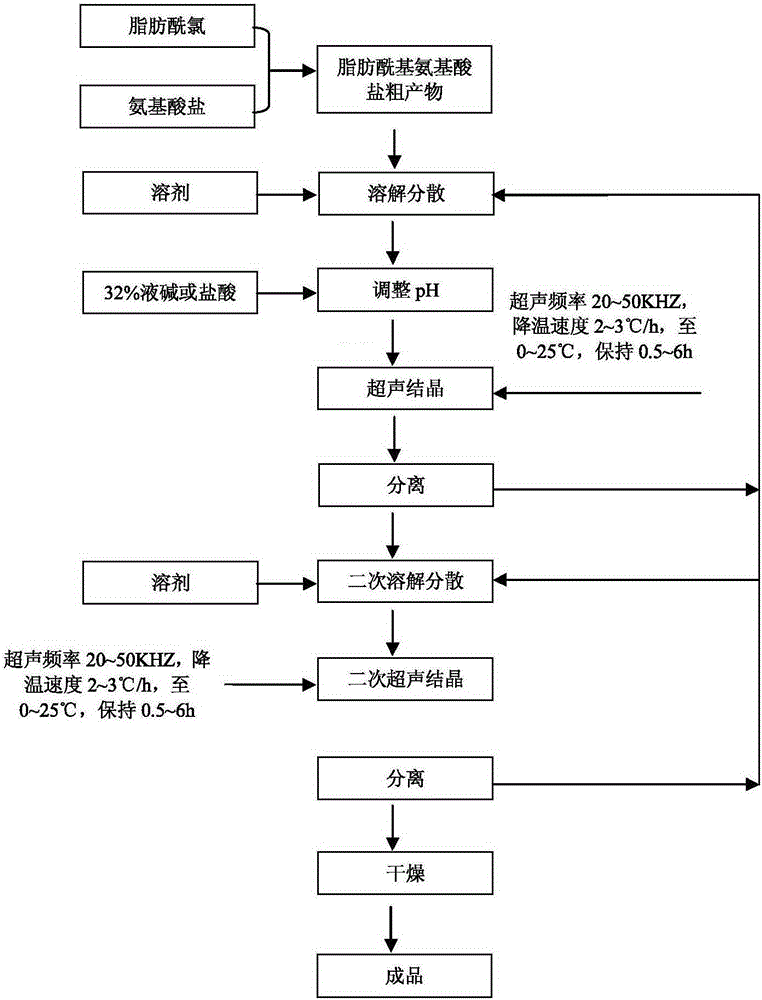
[0024] a) Synthesis of crude product: select methyl taurine and cocoyl chloride to react, 32% liquid caustic soda neutralizes the released hydrochloric acid, generate methyl cocoyl taurine sodium salt crude product, then cool down and crystallize, and centrifuge to obtain the content Sodium methyl cocoyl taurate powder with low salt content above 90%;
[0025] b) Dissolving and dispersing the crude product: 99.97g sodium methyl cocoyl taurate powder (among them, 90.3g sodium methyl cocoyl taurate, 5.45g water, 1.86g sodium chloride, sodium cocoate 2.03g, 0.32g of sodium methyl taurate, 0.0002g of iron, 0.000015g of chromium, 0.0035g of methylamine) with 1000g of 95% ethanol, heating and dissolving at 30-50°C, and adjusting the pH value to 7-9;
[0026] c) Ultrasonic crystallization for the first time: Turn on the ultrasound, frequency 15KHZ, slowly cool down (cooling rate 2-3°C / h), keep it at 20-25°C for 2 hours, observe through a microscope a small piece with good crystal sha...
Embodiment 2
[0032] a) Synthesis of crude product: select L-glutamic acid to react with lauroyl chloride, 32% liquid caustic soda neutralizes the released hydrochloric acid to generate sodium lauroyl glutamate;
[0033]b) Dissolving and dispersing the crude product: 100g of sodium lauroyl glutamate solution (among them, 31.42g of sodium lauroyl glutamate, 59.6g of water, 5.14g of sodium chloride, 1.98g of sodium laurate, and 1.98g of sodium L-glutamate 1.86g, iron 0.0002g, chromium 0.000015g, methylamine 0.0035g) with 300g of absolute ethanol, heated and dissolved at 30-50°C, and adjusted the pH value of the system to 5-6 with 1:1 hydrochloric acid;
[0034] c) Ultrasonic crystallization for the first time: turn on the ultrasound, the frequency is 65KHZ, slowly cool down (cooling rate 2-3°C / h), to 20-25°C, keep for 2 hours, observe through the microscope a small piece with good crystal shape and uniform particle size The crystals were filtered under reduced pressure, and 33.5 g of the filt...
Embodiment 3
[0040] a) Synthesis of crude product: react sarcosine with myristoyl chloride, neutralize the released hydrochloric acid with 48% KOH, and obtain the crude product of myristoyl sarcosinate potassium solution;
[0041] b) Dissolving and dispersing the crude product: 150.07g potassium myristoyl sarcosinate (among them, potassium myristoyl sarcosinate 38.86g, water 94.39g, potassium chloride 10.67g, potassium myristate 3.78g, sarcosine Potassium 2.37g, iron 0.0002g, chromium 0.000015g, methylamine 0.0035g) with 500g of absolute ethanol, heated and dissolved at 30-50°C to make a solution, and the measured pH value was 9-10;
[0042] c) Ultrasonic crystallization for the first time: Turn on the ultrasound, the frequency is 45KHZ, slowly cool down (cooling rate 2-3°C / h), and keep it at 20-25°C for 2 hours. Observing through the microscope, small flakes with good crystal shape and uniform particle size The crystals were vacuum filtered, and 40.91 g of the filter cake was collected fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com