Para nitro toluylene near ultraviolet photosencitizer, synthesis and uses thereof
A technology of nitrostilbene and near-ultraviolet light, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as inability to realize near-ultraviolet photopolymerization, and achieve convenient and easy source of raw materials The effect of obtaining and yield is suitable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
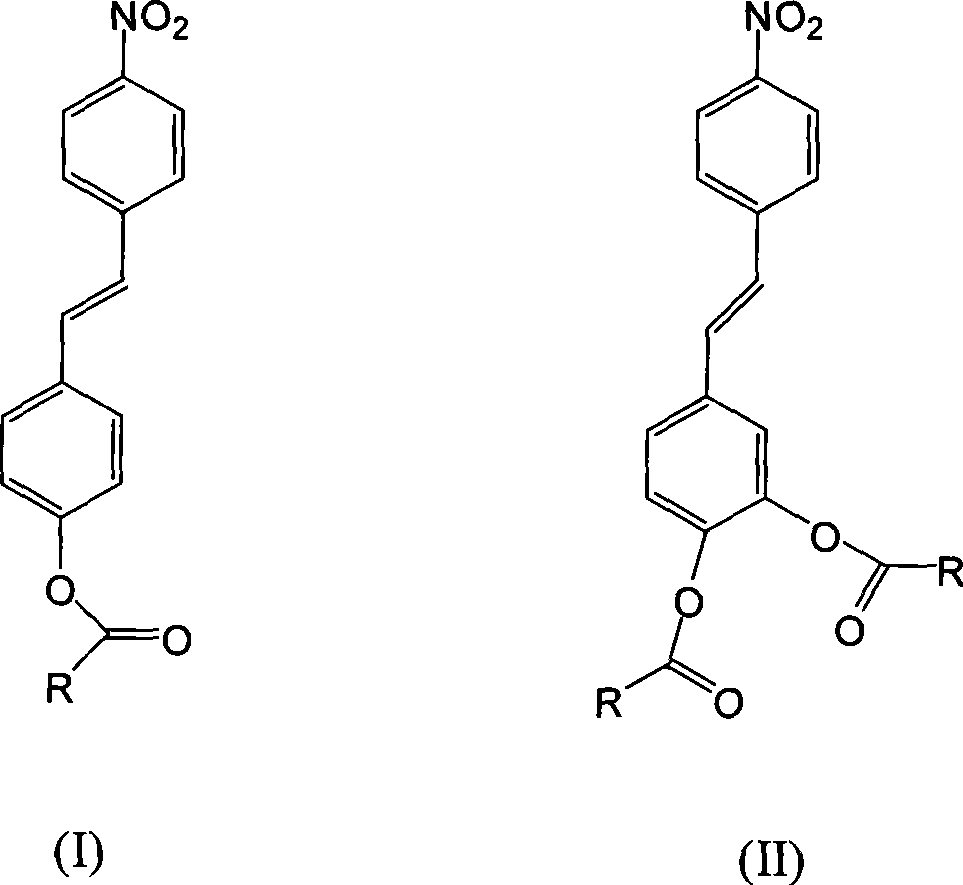
[0033] (Diacetic acid)-(p-nitrostilbene)-ester is synthesized in two steps:
[0034] (1) Synthesis of 3,4-dihydroxy-p-nitrostilbene
[0035] Add compound p-nitrophenylacetic acid (5.0g), 3,4-dihydroxybenzaldehyde (5.7g) and hexahydropyridine (4.1ml) in a three-necked flask (p-nitrophenylacetic acid: 3,4-dihydroxybenzene Formaldehyde: Hexahydropyridine (1:1.5:1.5) mixed, heated to 100°C and refluxed for 3-4 hours, until no bubbles were generated. Then the temperature was raised to 130° C., and the reaction was continued for 2 hours. The reactant was recrystallized twice from hot absolute ethanol to obtain the target compound (5.0 g) with a yield of 70%.
[0036] (2) Synthesis of (diacetic acid)-(p-nitrostilbene)-ester
[0037] Add 3,4-dihydroxy-p-nitrostilbene (1.5g), acetic anhydride (3.3ml), triethylamine (5.8ml) into the three-necked flask (3,4-dihydroxy-p-nitrostilbene : acetic anhydride: triethylamine = 1:6:8) mixed, add a condensing device, then dissolve with 150ml of...
Embodiment 2
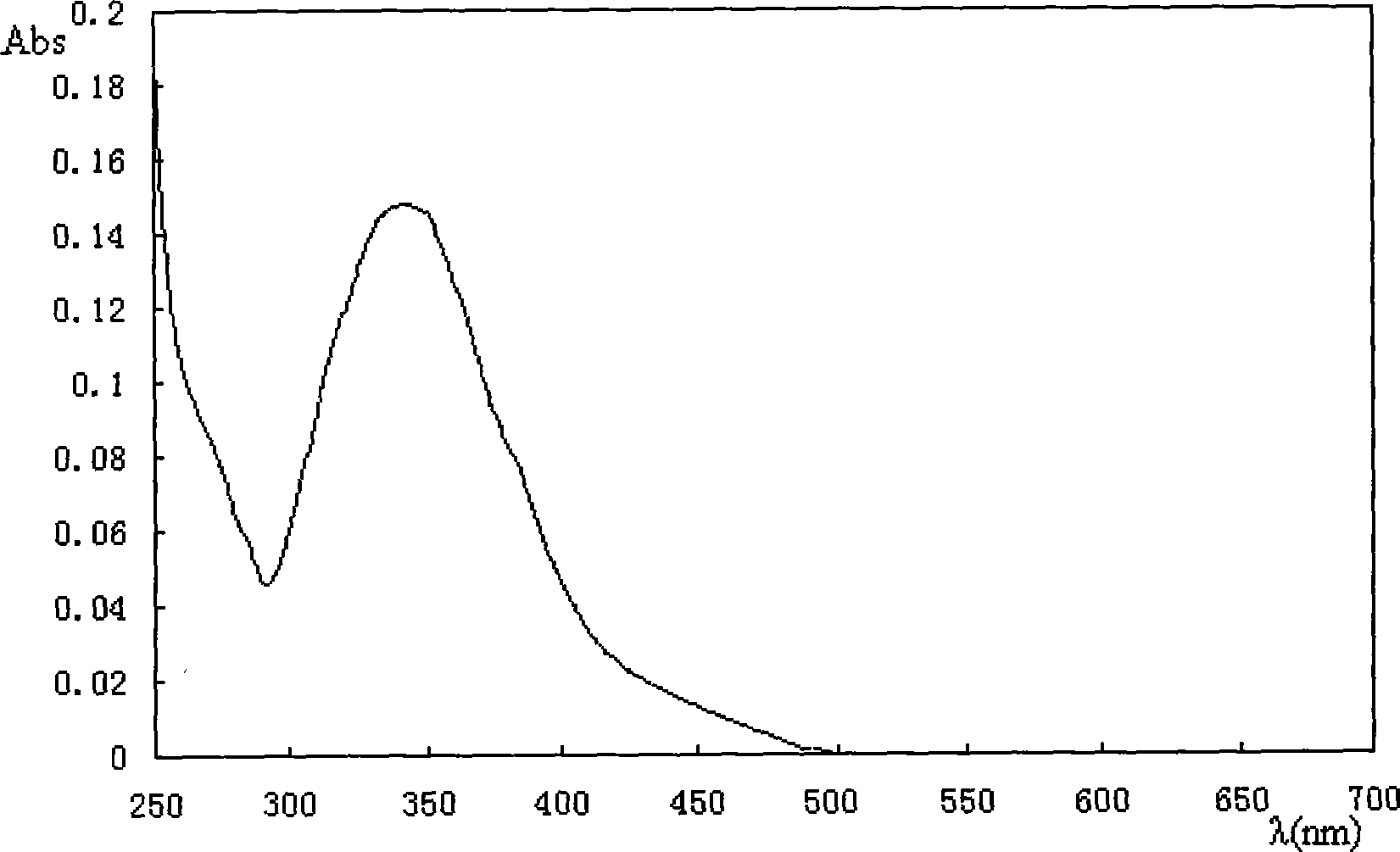
[0039] Synthesis of (dibenzoic acid)-(p-nitrostilbene)-ester
[0040] The synthesis proceeds in two steps
[0041] (1) Synthesis of 3,4-dihydroxy-p-nitrostilbene
[0042] Synthesis is carried out by step (1) in the implementation case 1.
[0043] (2) Synthesis of (dibenzoic acid)-(p-nitrostilbene)-ester
[0044] In the three-necked flask, add 3,4-dihydroxy-p-nitrostilbene (1.5g), benzoyl chloride (4.1ml), triethylamine (5.8ml) (3,4-dihydroxy-p-nitrostilbene Ethylene: benzoyl chloride: triethylamine = 1:6:8) mixed, add a condensing device, then dissolve with 150ml of dry tetrahydrofuran, reflux for 24 hours. After the reaction was completed, the precipitate was filtered off, and the crude product was obtained by passing through the column with dichloromethane, and recrystallized from benzene to obtain the target compound (1.1 g) as a yellow crystal, with a yield of 40%.
Embodiment 3
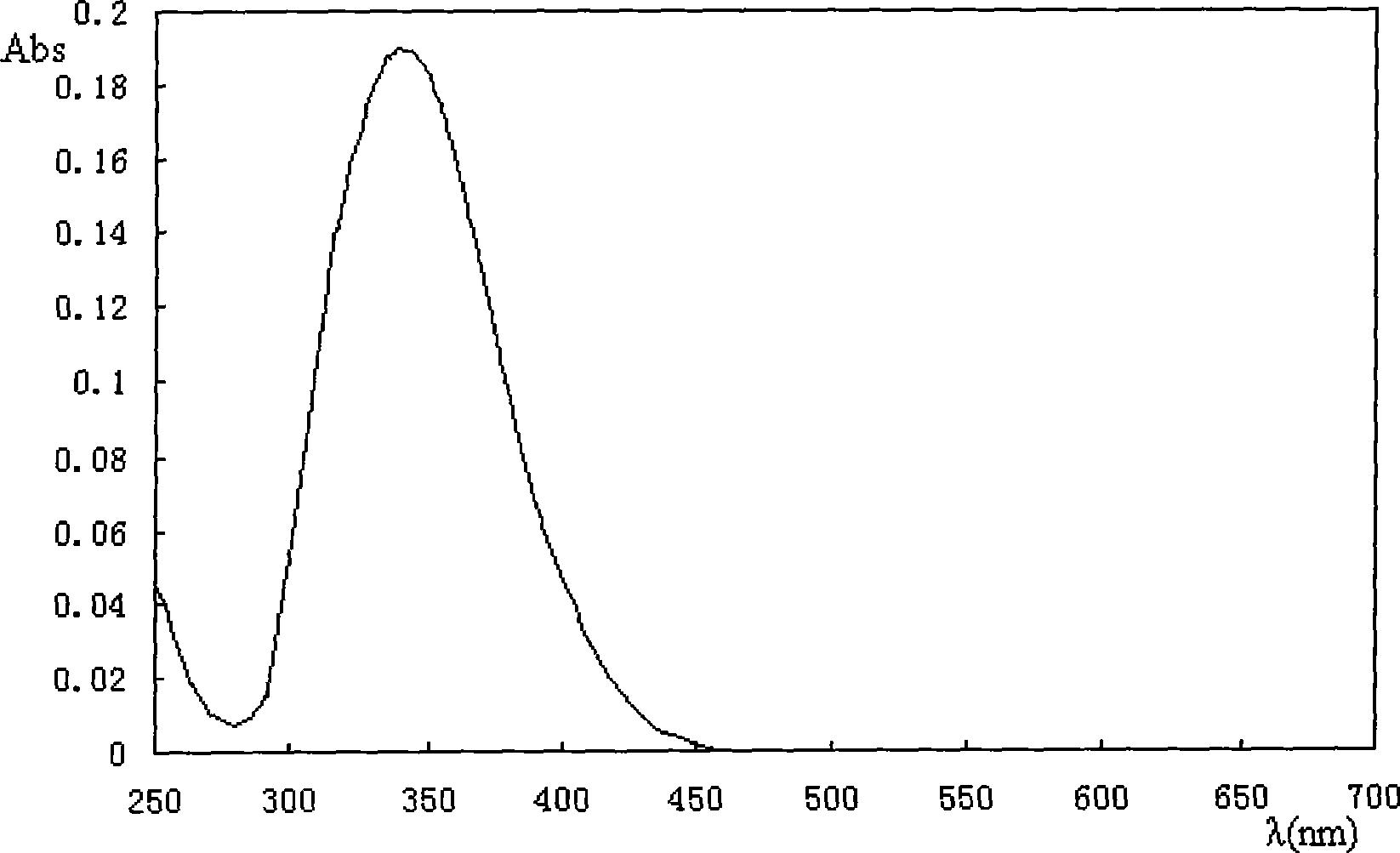
[0046] Will 1×10 -5 The (diacetic acid)-(p-nitrostilbene)-ester of mol / L is dissolved in methylene chloride, and its ultraviolet-visible absorption spectrum is measured, and its maximum absorption is at 337nm, such as figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com