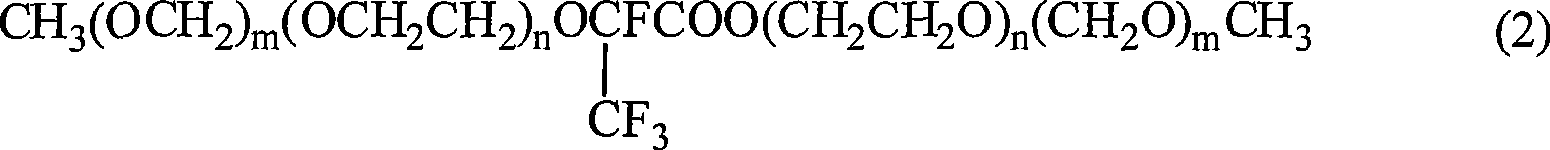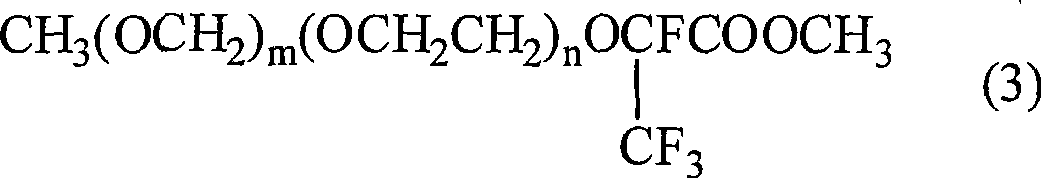Method for preparing perfluoro olefin ether from compound containing acid-sensing group
A perfluoroalkene ether compound technology, applied in the field of perfluoroalkene ether compounds, can solve problems such as decomposition and chain scission, difficulty in fluorination, low reaction yield, etc., achieve enhanced stability, reduce decomposition and chain scission, Good effect of perfluorinated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 250ml stainless steel reactor, add 1mol of CH 3 OCH 2 OCH 2 CH 2 OH and 1.5molNaF, stirred at room temperature, hexafluoropropylene oxide (HFPO) was passed into the reaction system in the form of bubbling from one outlet of the reactor, and its feed amount was based on the fact that the product content no longer increased, and the other The outlet is connected to a condenser, and the condenser is connected to a cold trap to cool the unreacted HFPO, and the cooled HFPO can be recycled. The reaction process was monitored by gas chromatography until the product content no longer increased, and the reaction was stopped. The reaction product was washed with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and distilled to obtain 0.35mol CH 3 OCH 2 OCH 2 CH 2 OCF (CF 3 ) COOCH 2 CH 2 OCH 2 OCH 3 , yield 70%. structure 1 HNMR, 19 Identification by F NMR and MS spectra.
Embodiment 2
[0039] In a 250ml stainless steel reactor, add 1mol of CH 3 OCH 2 (OCH 2 CH 2 ) 2 OH and 1.5molNaF, stirred at room temperature, passed into HFPO, the amount of its feed was based on the fact that the product content would no longer increase, and the unreacted HFPO was cooled down with a cold trap. The reaction process was monitored by gas chromatography until the product content no longer increased, and the reaction was stopped. The reaction product was processed in a similar manner to Example 1 to obtain 0.33molCH 3 OCH 2 (OCH 2 CH 2 ) 2 OCF (CF 3 )COO(CH 2 CH 2 O) 2 CH 2 OCH 3 , yield 65%. structure 1 HNMR, 19 Identification by F NMR and MS spectra.
Embodiment 3
[0041] In a 2L stainless steel reactor, add 20mol methanol, 0.12mol sodium hydroxide, stir at room temperature, after the alkali is dissolved, add 1mol CH 3 OCH 2 OCH 2 CH 2 OCF (CF 3 ) COOCH 2 CH 2 OCH 2 OCH 3 , heated to reflux, and reacted for 30 hours. The reaction process was monitored by gas chromatography until the product content no longer increased, and the reaction was stopped. The methanol was evaporated, the reaction product was washed with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and distilled to obtain 0.95mol CH 3 OCH 2 OCH 2 CH 2 OCF (CF 3 ) COOCH 3 , 95% yield. Collect distillate at the same time, recover HOCH 2 CH 2 OCH 2 OCH 3 . structure 1 H NMR, 19 Identification by F NMR and MS spectra.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com